Obstet Gynecol Sci.
2020 Sep;63(5):631-642. 10.5468/ogs.20049.
Risk factors for type-specific persistence of high-risk human papillomavirus and residual/recurrent cervical intraepithelial neoplasia after surgical treatment
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 2Department of Obstetrics and Gynecology, Graduate School of Medicine, Kangwon National University, Chuncheon, Korea
- 3Gynecologic Cancer Center, Department of Obstetrics and Gynecology, CHA University Ilsan Medical Center, Goyang, Korea
- 4Department of Obstetrics and Gynecology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 5Department of Obstetrics and Gynecology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2506506
- DOI: http://doi.org/10.5468/ogs.20049
Abstract
Objective
This study aimed to investigate the clinicopathologic risk factors for type-specific persistence of high-risk human papillomavirus (hrHPV) and residual/recurrent cervical intraepithelial neoplasia (CIN) after surgical treatment.
Methods
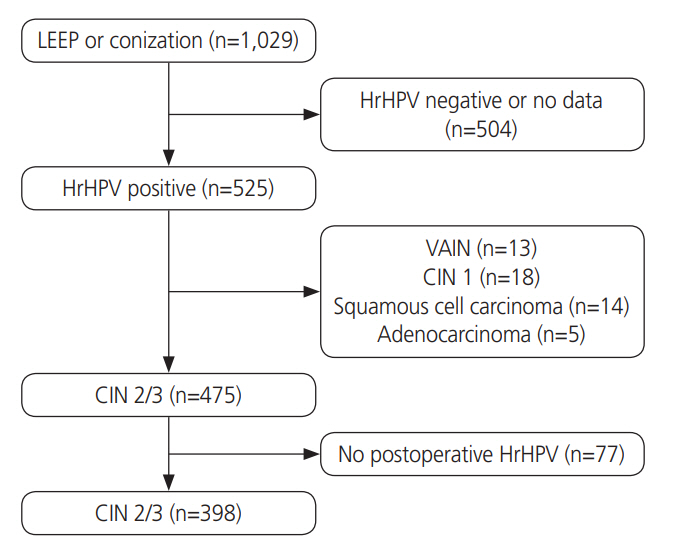
Patients with CIN-2/3 who underwent conization or loop electrosurgical excision procedure (LEEP) at Korea University Hospital were enrolled. All patients underwent hrHPV testing and genotyping before conization or LEEP followed by both hrHPV genotyping and cytology. The significance of associations between patient characteristics and persistence of infection were assessed by multivariate logistic regression analyses.
Results
Among 398 women with pathologically confirmed CIN-2/3, 154 (38.7%) patients showed hrHPV persistence after surgical treatment. In multivariate analysis, high preoperative hrHPV load (P<0.05; odds ratio [OR], 2.063), presence of CIN-2 at treatment (P<0.01; OR, 2.732), and multiple hrHPV infections (P<0.001; OR, 4.752) were associated with hrHPV persistence. HPV 53 was the most likely to persist after treatment (24/43, 55.8%). The risk of residual/recurrent CIN-2/3 was higher in persistent infection with HPV 16 than other types (P<0.05). Menopause (P<0.001; OR, 3.969), preoperative and postoperative hrHPV load (P<0.05; OR, 2.430; P<0.05; OR, 5.351), and infection with multiple hrHPV types (P<0.05; OR, 2.345) were significantly related to residual/recurrent CIN following surgical treatment.
Conclusion
HPV load before treatment and infection with multiple hrHPV types were predictors of postoperative hrHPV persistence. HPV 53 was the type most likely to persist, but HPV 16 was the type that was most closely associated with residual/recurrent CIN-2/3.
Figure
Cited by 1 articles
-
Impact of hematologic toxicities during concurrent chemoradiation for cervical cancer
Feiya Shi, Alison K. Yoder, Claire Mach, Shraddha Dalwadi, Matthew L Anderson, Tracilyn R Hall, Michelle S Ludwig
Obstet Gynecol Sci. 2022;65(2):176-187. doi: 10.5468/ogs.21308.
Reference
-
References
1. Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005; 337:76–84.
Article2. Del Río-Ospina L, Soto-DE León SC, Camargo M, Sánchez R, Moreno-Pérez DA, Pérez-Prados A, et al. Multiple high-risk HPV genotypes are grouped by type and are associated with viral load and risk factors. Epidemiol Infect. 2017; 145:1479–90.3. Sarian LO, Derchain SF, Pitta DR, Morais SS, RabeloSantos SH. Factors associated with HPV persistence after treatment for high-grade cervical intra-epithelial neoplasia with large loop excision of the transformation zone (LLETZ). J Clin Virol. 2004; 31:270–4.
Article4. Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013; 121:829–46.
Article5. Melnikow J, McGahan C, Sawaya GF, Ehlen T, Coldman A. Cervical intraepithelial neoplasia outcomes after treatment: long-term follow-up from the British Columbia Cohort Study. J Natl Cancer Inst. 2009; 101:721–8.
Article6. Venturoli S, Ambretti S, Cricca M, Leo E, Costa S, Musiani M, et al. Correlation of high-risk human papillomavirus genotypes persistence and risk of residual or recurrent cervical disease after surgical treatment. J Med Virol. 2008; 80:1434–40.
Article7. Alonso I, Torné A, Puig-Tintoré LM, Esteve R, Quinto L, Campo E, et al. Pre- and post-conization high-risk HPV testing predicts residual/recurrent disease in patients treated for CIN 2-3. Gynecol Oncol. 2006; 103:631–6.
Article8. Kocken M, Uijterwaal MH, de Vries AL, Berkhof J, Ket JC, Helmerhorst TJ, et al. High-risk human papillomavirus testing versus cytology in predicting post-treatment disease in women treated for high-grade cervical disease: a systematic review and meta-analysis. Gynecol Oncol. 2012; 125:500–7.
Article9. Kitchener HC, Walker PG, Nelson L, Hadwin R, Patnick J, Anthony GB, et al. HPV testing as an adjunct to cytology in the follow up of women treated for cervical intraepithelial neoplasia. BJOG. 2008; 115:1001–7.
Article10. Kocken M, Helmerhorst TJ, Berkhof J, Louwers JA, Nobbenhuis MA, Bais AG, et al. Risk of recurrent highgrade cervical intraepithelial neoplasia after successful treatment: a long-term multi-cohort study. Lancet Oncol. 2011; 12:441–50.
Article11. Kim YT, Lee JM, Hur SY, Cho CH, Kim YT, Kim SC, et al. Clearance of human papillomavirus infection after successful conization in patients with cervical intraepithelial neoplasia. Int J Cancer. 2010; 126:1903–9.
Article12. Aerssens A, Claeys P, Beerens E, Garcia A, Weyers S, Van Renterghem L, et al. Prediction of recurrent disease by cytology and HPV testing after treatment of cervical intraepithelial neoplasia. Cytopathology. 2009; 20:27–35.
Article13. Lubrano A, Medina N, Benito V, Arencibia O, Falcón JM, Leon L, et al. Follow-up after LLETZ: a study of 682 cases of CIN 2-CIN 3 in a single institution. Eur J Obstet Gynecol Reprod Biol. 2012; 161:71–4.
Article14. Song SH, Lee JK, Oh MJ, Hur JY, Na JY, Park YK, et al. Persistent HPV infection after conization in patients with negative margins. Gynecol Oncol. 2006; 101:418–22.
Article15. Gosvig CF, Huusom LD, Andersen KK, Iftner A, Cederkvist L, Svare E, et al. Persistence and reappearance of high-risk human papillomavirus after conization. Gynecol Oncol. 2013; 131:661–6.
Article16. Kwon MJ, Roh KH, Park H, Woo HY. Comparison of the Anyplex II HPV28 assay with the Hybrid Capture 2 assay for the detection of HPV infection. J Clin Virol. 2014; 59:246–9.
Article17. Phillips S, Cornall AM, Machalek DA, Garland SM, Bateson D, Garefalakis M, et al. Comparison of the Roche Cobas® 4800 HPV assay to Roche Amplicor for detection of high-risk human papillomavirus. Eur J Clin Microbiol Infect Dis. 2016; 35:1305–7.
Article18. Jung S, Lee B, Lee KN, Kim Y, Oh EJ. Clinical validation of Anyplex II HPV HR detection test for cervical cancer screening in Korea. Arch Pathol Lab Med. 2016; 140:276–80.
Article19. Costa S, De Simone P, Venturoli S, Cricca M, Zerbini ML, Musiani M, et al. Factors predicting human papillomavirus clearance in cervical intraepithelial neoplasia lesions treated by conization. Gynecol Oncol. 2003; 90:358–65.
Article20. Stensen S, Kjaer SK, Jensen SM, Frederiksen K, Junge J, Iftner T, et al. Factors associated with type-specific persistence of high-risk human papillomavirus infection: A population-based study. Int J Cancer. 2016; 138:361–8.
Article21. Kong TW, Son JH, Chang SJ, Paek J, Lee Y, Ryu HS. Value of endocervical margin and high-risk human papillomavirus status after conization for high-grade cervical intraepithelial neoplasia, adenocarcinoma in situ, and microinvasive carcinoma of the uterine cervix. Gynecol Oncol. 2014; 135:468–73.
Article22. Dalstein V, Riethmuller D, Prétet JL, Le Bail Carval K, Sautière JL, Carbillet JP, et al. Persistence and load of highrisk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003; 106:396–403.
Article23. Bae JH, Kim CJ, Park TC, Namkoong SE, Park JS. Persistence of human papillomavirus as a predictor for treatment failure after loop electrosurgical excision procedure. Int J Gynecol Cancer. 2007; 17:1271–7.
Article24. Schmitt M, Dondog B, Waterboer T, Pawlita M, Tommasino M, Gheit T. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J Clin Microbiol. 2010; 48:143–9.
Article25. Chaturvedi AK, Katki HA, Hildesheim A, Rodríguez AC, Quint W, Schiffman M, et al. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis. 2011; 203:910–20.
Article26. Sherman ME, Wang SS, Wheeler CM, Rich L, Gravitt PE, Tarone R, et al. Determinants of human papillomavirus load among women with histological cervical intraepithelial neoplasia 3: dominant impact of surrounding low-grade lesions. Cancer Epidemiol Biomarkers Prev. 2003; 12:1038–44.27. Papoutsis D, Underwood M, Parry-Smith W, Panikkar J. Does CIN2 have the same aggressive potential as CIN3? A Secondary analysis of high-grade cytology recurrence in women treated with cold-coagulation cervical treatment. Geburtshilfe Frauenheilkd. 2017; 77:284–9.
Article28. Pirtea L, Grigoraş D, Matusz P, Pirtea M, Moleriu L, Tudor A, et al. Age and HPV type as risk factors for HPV persistence after loop excision in patients with high grade cervical lesions: an observational study. BMC Surg. 2016; 16:70.
Article29. Rizzuto I, Nalam M, Jiang J, Linder A, Rufford B. Risk factors for HPV persistence and cytology anomalies at follow-up after treatment for cervical dysplasia. Int J Gynaecol Obstet. 2018; 141:240–4.
Article30. So KA, Lee IH, Lee KH, Hong SR, Kim YJ, Seo HH, et al. Human papillomavirus genotype-specific risk in cervical carcinogenesis. J Gynecol Oncol. 2019; 30:e52.
Article31. Aho J, Hankins C, Tremblay C, Forest P, Pourreaux K, Rouah F, et al. Genomic polymorphism of human papillomavirus type 52 predisposes toward persistent infection in sexually active women. J Infect Dis. 2004; 190:46–52.
Article32. Kavanagh K, Pollock KG, Potts A, Love J, Cuschieri K, Cubie H, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. 2014; 110:2804–11.
Article33. So KA, Hong JH, Lee JK. Human papillomavirus prevalence and type distribution among 968 women in South Korea. J Cancer Prev. 2016; 21:104–9.
Article34. Kang WD, Kim SM. Human papillomavirus genotyping as a reliable prognostic marker of recurrence after loop electrosurgical excision procedure for high-grade cervical intraepithelial neoplasia (CIN2-3) especially in postmenopausal women. Menopause. 2016; 23:81–6.
Article35. Söderlund-Strand A, Kjellberg L, Dillner J. Human papillomavirus type-specific persistence and recurrence after treatment for cervical dysplasia. J Med Virol. 2014; 86:634–41.
Article36. Kreimer AR, Katki HA, Schiffman M, Wheeler CM, Castle PE; ASCUS-LSIL Triage Study Group. Viral determinants of human papillomavirus persistence following loop electrical excision procedure treatment for cervical intraepithelial neoplasia grade 2 or 3. Cancer Epidemiol Biomarkers Prev. 2007; 16:11–6.
Article37. Nam K, Chung S, Kim J, Jeon S, Bae D. Factors associated with HPV persistence after conization in patients with negative margins. J Gynecol Oncol. 2009; 20:91–5.
Article38. Park JY, Lee KH, Dong SM, Kang S, Park SY, Seo SS. The association of pre-conization high-risk HPV load and the persistence of HPV infection and persistence/recurrence of cervical intraepithelial neoplasia after conization. Gynecol Oncol. 2008; 108:549–54.
Article39. Moscicki AB, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012; 30 Suppl 5:F24–33.
Article40. Elfgren K, Jacobs M, Walboomers JM, Meijer CJ, Dillner J. Rate of human papillomavirus clearance after treatment of cervical intraepithelial neoplasia. Obstet Gynecol. 2002; 100:965–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Persistence of HPV after LEEP Treatment in Cervical Intraepithelial Neoplasia
- Type-specific persistence or regression of human papillomavirus genotypes in women with cervical intraepithelial neoplasia 1: A prospective cohort study
- Posttreatment human papillomavirus testing for residual or recurrent high-grade cervical intraepithelial neoplasia: a pooled analysis
- Detection of Human Papillomavius DNA by Hybrid Capture Test in Cervical Intraepithelial Neoplasia and Carcinoma
- Effect of human papillomavirus genotype on severity and prognosis of cervical intraepithelial neoplasia