J Korean Med Sci.
2020 Jul;35(29):e235. 10.3346/jkms.2020.35.e235.
Similar Durability of Two Single Tablet Regimens, Dolutegravir/Abacavir/Lamivudine and Elvitegravir/Cobicistat/Tenofovir/Emtricitabine: Single Center Experience
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, National Medical Center, Seoul, Korea
- 2Laboratory for Future Emergency Medical Service, National Medical Center, Seoul, Korea
- KMID: 2504699
- DOI: http://doi.org/10.3346/jkms.2020.35.e235
Abstract
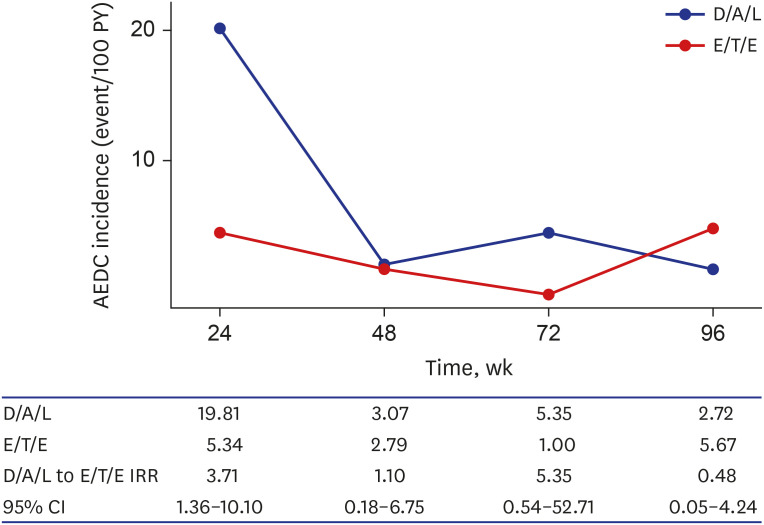
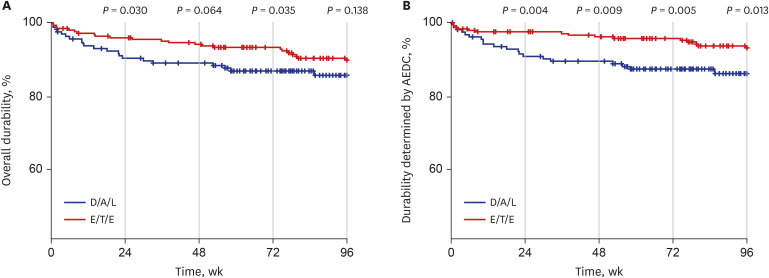
- Integrase inhibitor is uniquely available as single tablet regimen (STR) in Korea. In this study, the durability until 96 weeks was compared between dolutegravir/abacavir/lamivudine (D/A/L) and elvitegravir/cobicistat/tenofovir/emtricitabine (E/T/E) in treatment naïve human immunodeficiency virus 1 (HIV-1) infected individuals. From 2014 to 2017, 153 and 234 subjects started D/A/L and E/T/E, respectively. During 96 weeks, 73 discontinued initial STR and the reason of discontinuation was typable in 44. The frequency of drug adverse event related discontinuation (AEDC) was higher in D/A/L (13.1% vs. 6.4%, P = 0.023) while most non-AE related discontinuations occurred in E/T/E (8/9), such as drug-drug interaction, meal requirement and virologic failure. AEDC occurred usually within 24 weeks (20/35) and D/A/L to E/T/E AEDC incidence rate ratio was 3.71 (95% confidence interval, 1.36–10.10) in this period. Regarding the durability, D/A/L and E/T/E revealed no significant difference at week 96 (P = 0.138) while durability of D/A/L was worse in the aspect of AEDC (P = 0.013).
Figure
Reference
-
1. Daniel V, Süsal C, Melk A, Weimer R, Kröpelin M, Zimmermann R, et al. Reduction of viral load and immune complex load on CD4+ lymphocytes as a consequence of highly active antiretroviral treatment (HAART) in HIV-infected hemophilia patients. Immunol Lett. 1999; 69(2):283–289. PMID: 10482364.
Article2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Updated 2019. Accessed January 10, 2020. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.3. World Health Organization. Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva: World Health Organization;2018.4. European AIDS Clinical Society. EACS Guidelines 10.0. Brussels, Belgium: European AIDS Clinical Society;2019.5. Hazuda DJ, Felock P, Witmer M, Wolfe A, Stillmock K, Grobler JA, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000; 287(5453):646–650. PMID: 10649997.
Article6. Lampiris HW. Elvitegravir: a once-daily, boosted, HIV-1 integrase inhibitor. Expert Rev Anti Infect Ther. 2012; 10(1):13–20. PMID: 22149610.
Article7. Hemmige V, Flash CA, Carter J, Giordano TP, Zerai T. Single tablet HIV regimens facilitate virologic suppression and retention in care among treatment naïve patients. AIDS Care. 2018; 30(8):1017–1024. PMID: 29478329.
Article8. Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002; 359(9308):727–732. PMID: 11888582.9. Dorjee K, Choden T, Baxi SM, Steinmaus C, Reingold AL. Risk of cardiovascular disease associated with exposure to abacavir among individuals with HIV: A systematic review and meta-analyses of results from 17 epidemiologic studies. Int J Antimicrob Agents. 2018; 52(5):541–553. PMID: 30040992.
Article10. Dorjee K, Baxi SM, Reingold AL, Hubbard A. Risk of cardiovascular events from current, recent, and cumulative exposure to abacavir among persons living with HIV who were receiving antiretroviral therapy in the United States: a cohort study. BMC Infect Dis. 2017; 17(1):708. PMID: 29078761.
Article11. Cattaneo D, Baldelli S, Minisci D, Meraviglia P, Clementi E, Galli M, et al. When food can make the difference: The case of elvitegravir-based co-formulation. Int J Pharm. 2016; 512(1):301–304. PMID: 27592195.
Article12. Nguyen T, McNicholl I, Custodio JM, Szwarcberg J, Piontkowsky D. Drug interactions with cobicistat- or ritonavir-boosted elvitegravir. AIDS Rev. 2016; 18(2):101–111. PMID: 27196356.13. Castagna A, Maggiolo F, Penco G, Wright D, Mills A, Grossberg R, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014; 210(3):354–362. PMID: 24446523.
Article14. Todd S, Rafferty P, Walker E, Hunter M, Dinsmore WW, Donnelly CM, et al. Early clinical experience of dolutegravir in an HIV cohort in a larger teaching hospital. Int J STD AIDS. 2017; 28(11):1074–1081. PMID: 28118801.
Article15. Hoffmann C, Welz T, Sabranski M, Kolb M, Wolf E, Stellbrink HJ, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017; 18(1):56–63. PMID: 27860104.
Article16. Cid-Silva P, Llibre JM, Fernández-Bargiela N, Margusino-Framiñán L, Balboa-Barreiro V, Pernas-Souto B, et al. Clinical experience with the integrase inhibitors dolutegravir and elvitegravir in HIV-infected patients: efficacy, safety and tolerance. Basic Clin Pharmacol Toxicol. 2017; 121(5):442–446. PMID: 28627771.
Article17. de Boer MG, van den Berk GE, van Holten N, Oryszcyn JE, Dorama W, Moha DA, et al. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS. 2016; 30(18):2831–2834. PMID: 27824625.
Article18. Peñafiel J, de Lazzari E, Padilla M, Rojas J, Gonzalez-Cordon A, Blanco JL, et al. Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother. 2017; 72(6):1752–1759. PMID: 28333231.
Article19. Kanters S, Vitoria M, Doherty M, Socias ME, Ford N, Forrest JI, et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV. 2016; 3(11):e510–e520. PMID: 27658869.
Article20. Patel DA, Snedecor SJ, Tang WY, Sudharshan L, Lim JW, Cuffe R, et al. 48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1-infected patients: a systematic review and network meta-analysis. PLoS One. 2014; 9(9):e105653. PMID: 25188312.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Weight Gain and Lipid Profile Changes in Koreans with Human Immunodeficiency Virus undergoing Integrase Strand Transfer Inhibitor-Based Regimens
- Efficacy and Safety of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil Fumarate in Asian Subjects with Human Immunodeficiency Virus 1 Infection: A Sub-Analysis of Phase 3 Clinical Trials
- Angle's Class II Division 2 Malocclusion Treated by Bioprogressive Mechanism: Report of a Case
- A Korean Post-Marketing Study of Abacavir/Dolutegravir/Lamivudine in Patients with HIV-1
- Clinical and radiobiological consideration of cyclical hypofractionated radiation therapy also known as QUAD Shot for neglected skin cancer disfiguring the face of a non-compliant patient who was refusing surgery and protracted radiation therapy: case report