J Pathol Transl Med.
2020 Jul;54(4):318-331. 10.4132/jptm.2020.02.26.
Current status of cytopathology practices in Korea: annual report on the Continuous Quality Improvement program of the Korean Society for Cytopathology for 2018
- Affiliations
-
- 1Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Pathology, Eone Laboratories, Incheon, Korea
- 3Department of Pathology, Eulji University Hospital, Eulji University School of Medicine, Seoul, Korea
- 4Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Pathology, Daegu Catholic University School of Medicine, Daegu, Korea
- KMID: 2504562
- DOI: http://doi.org/10.4132/jptm.2020.02.26
Abstract
- Background
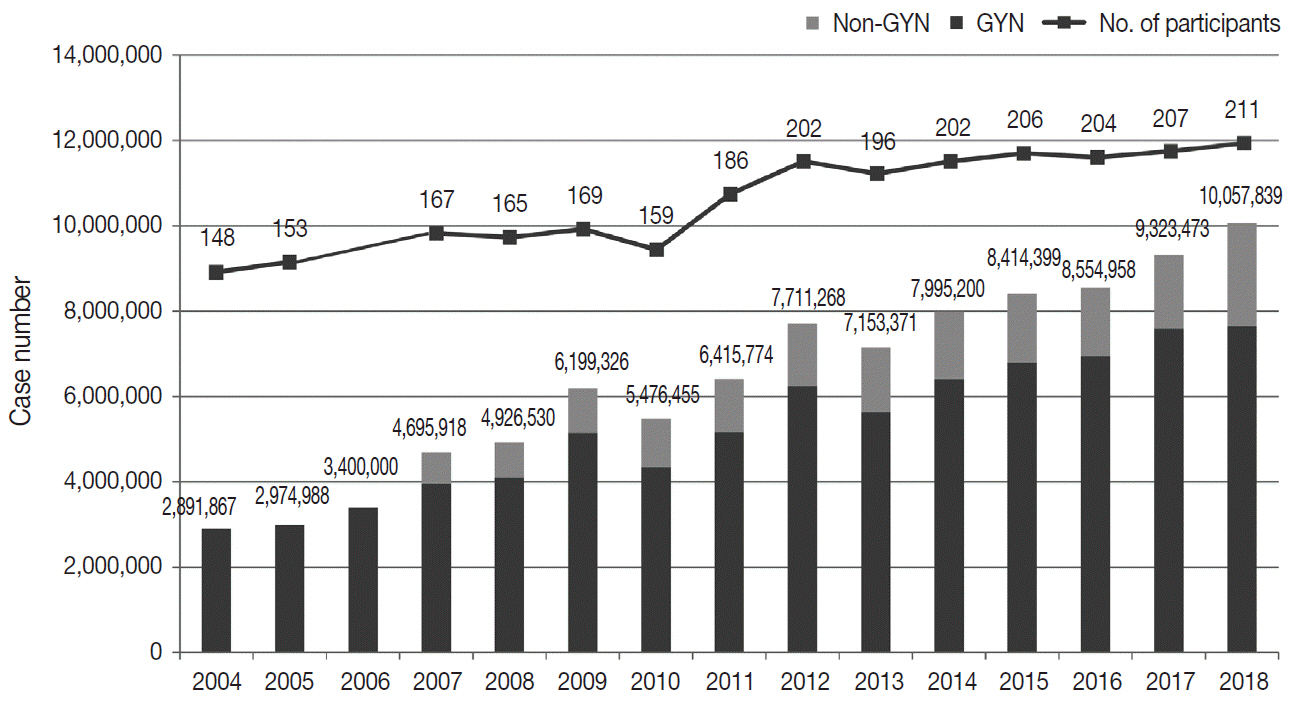
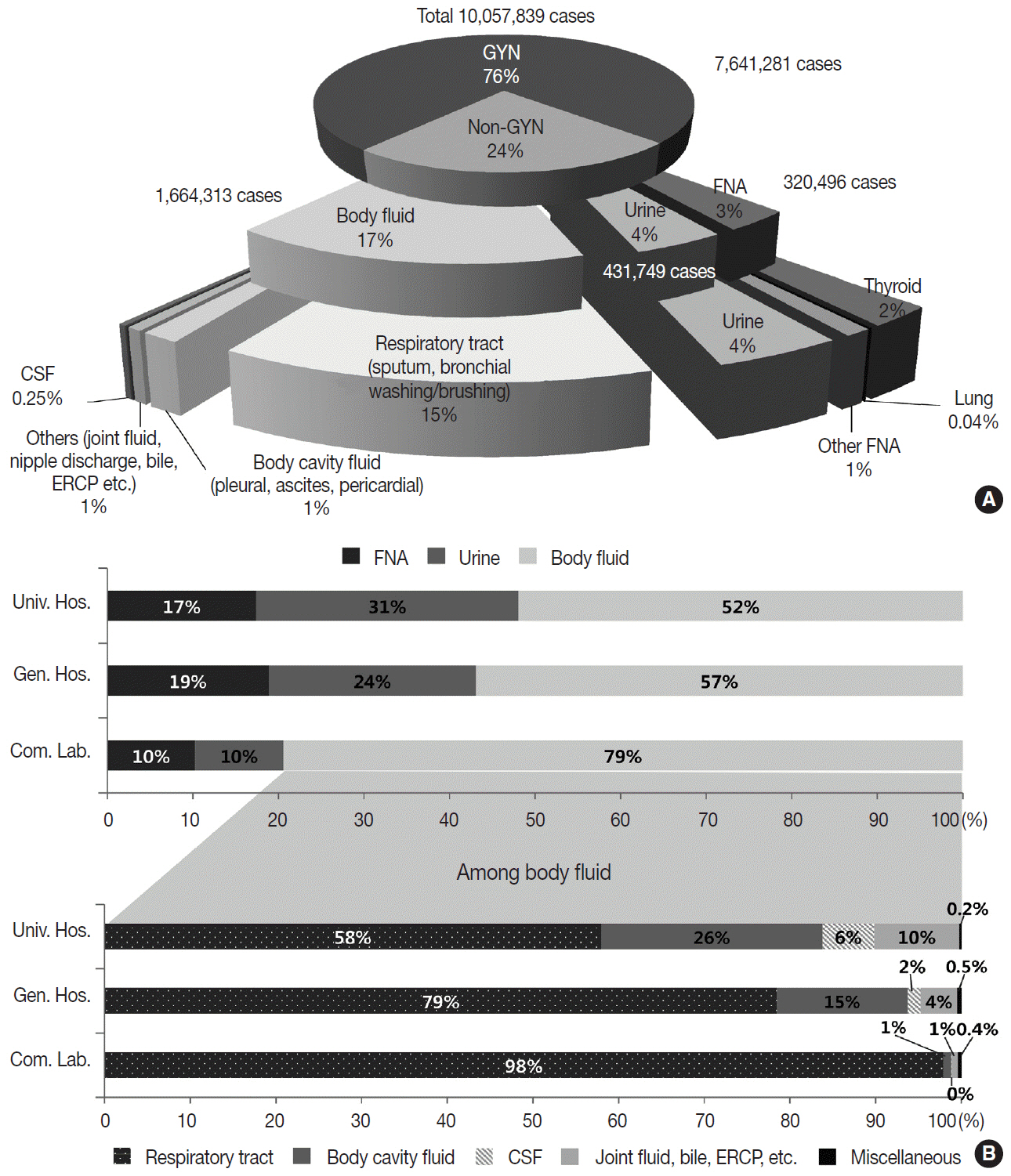
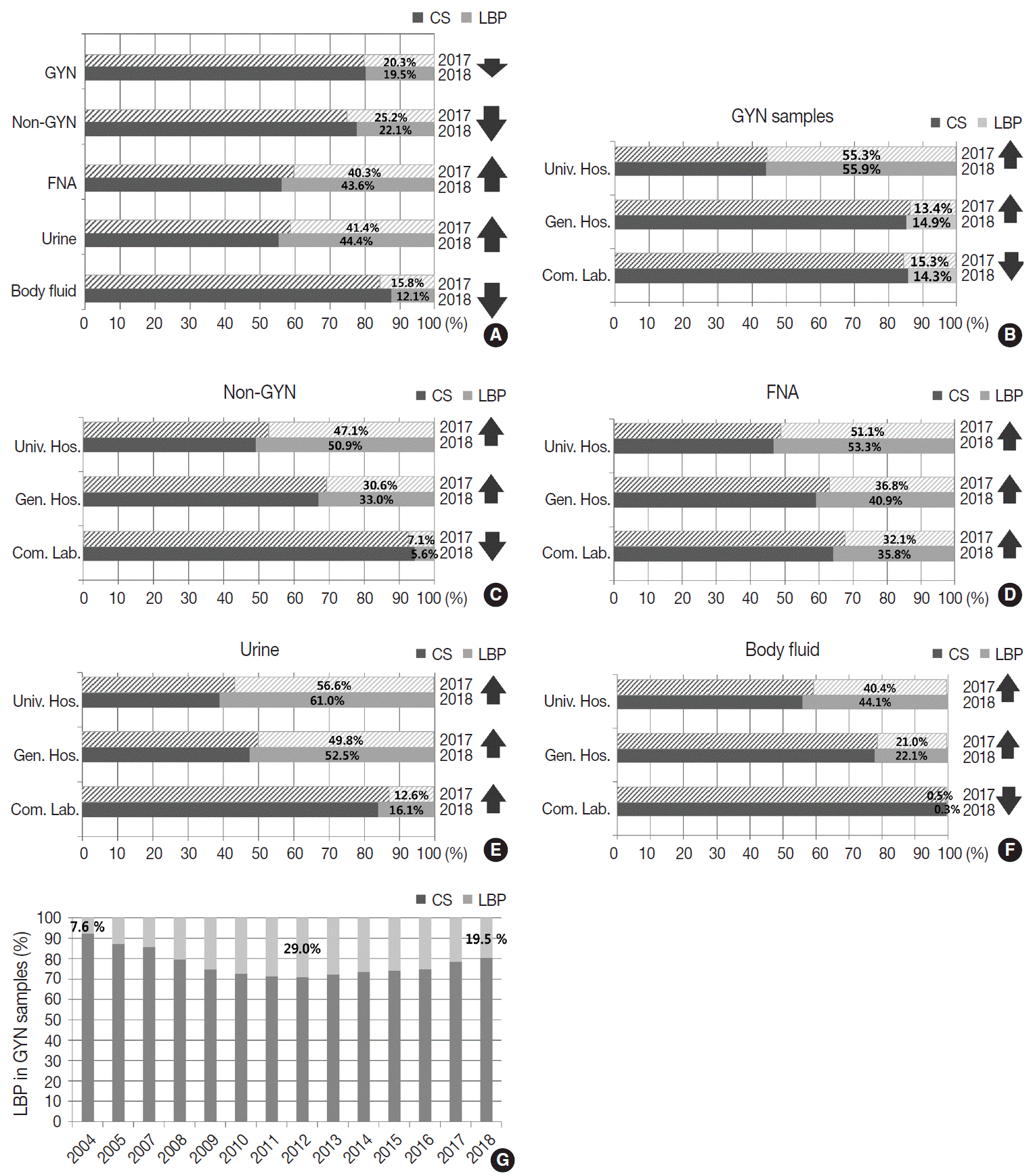
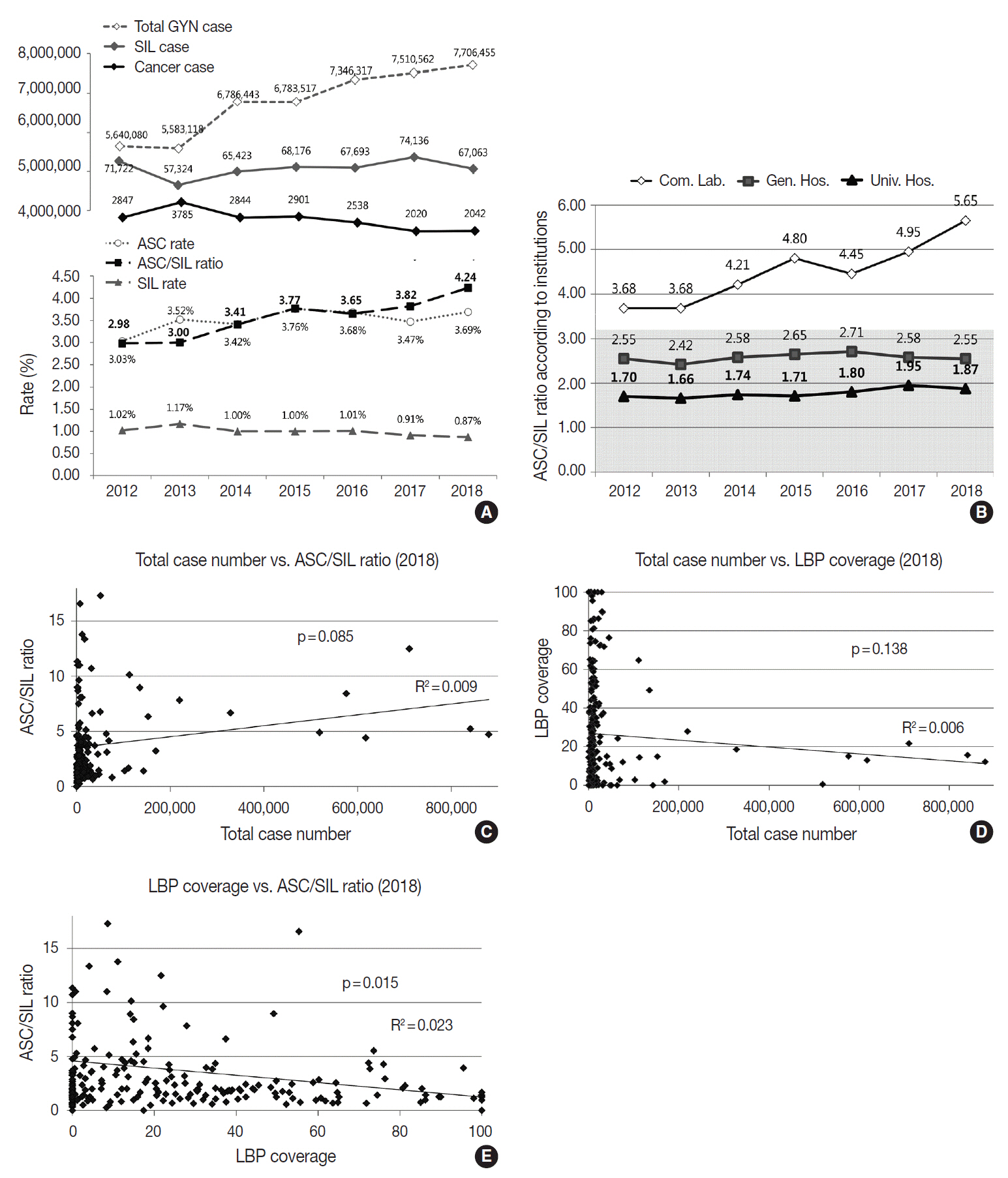
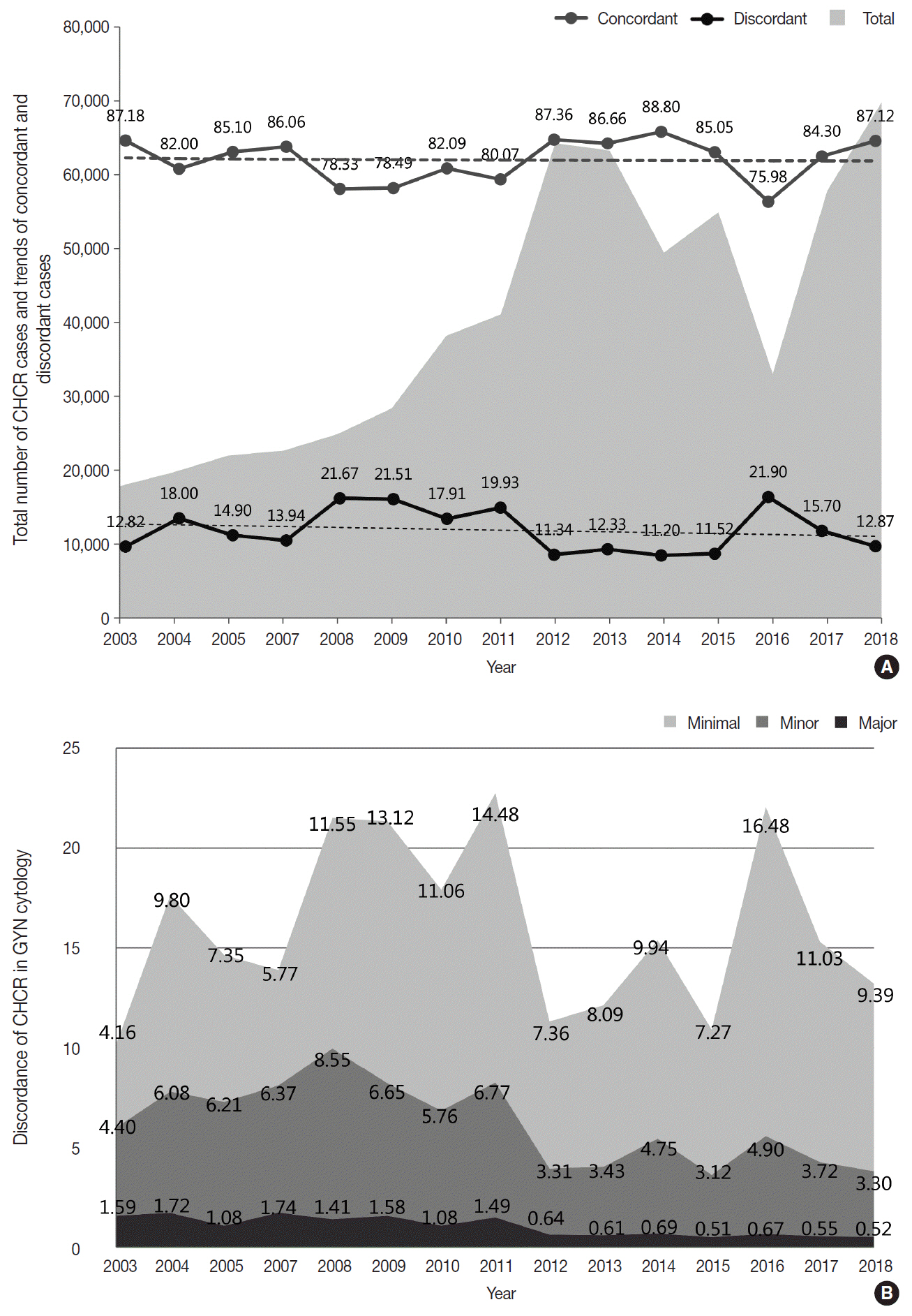
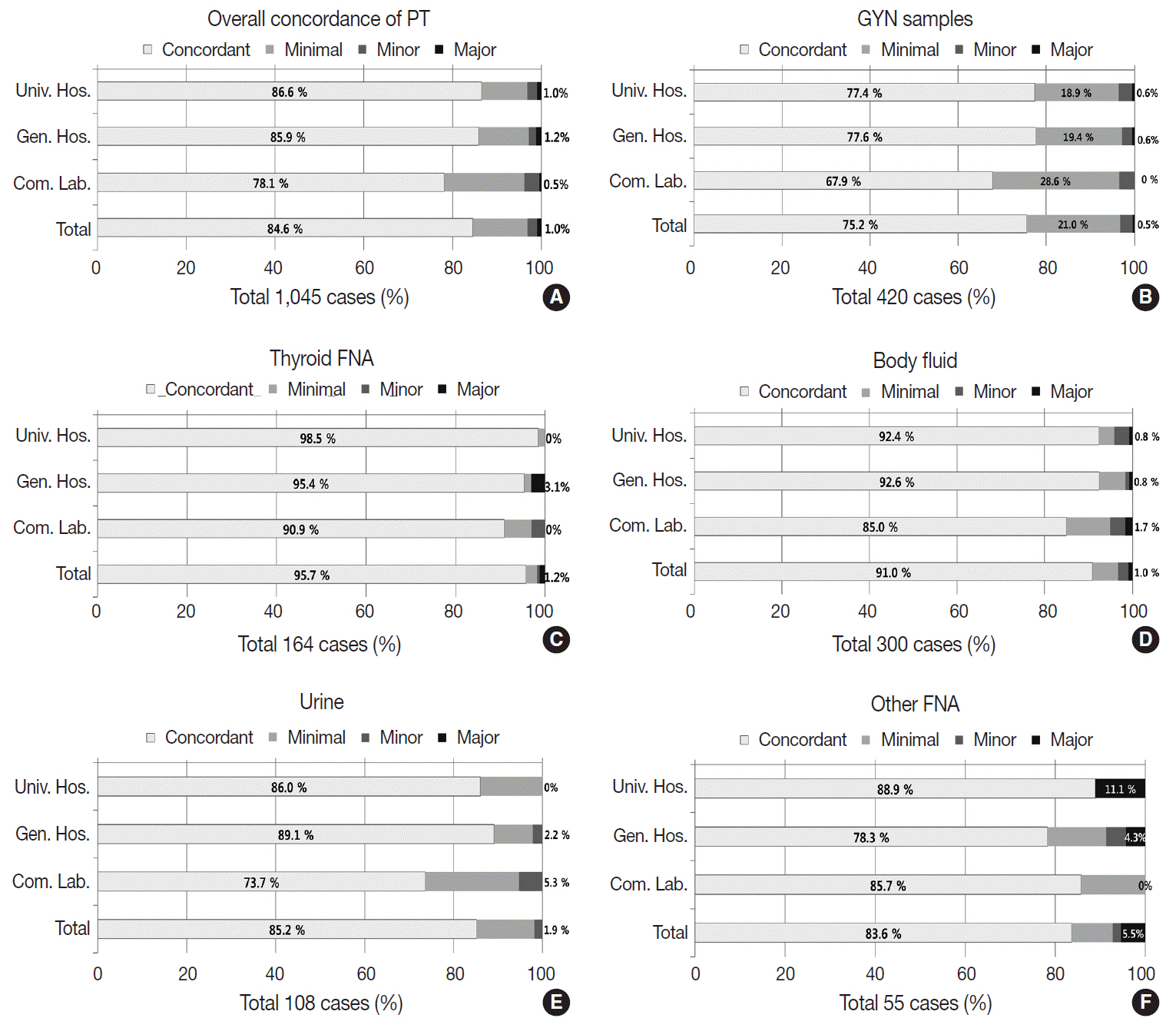
The Korean Society for Cytopathology has conducted the Continuous Quality Improvement program for cytopathology laboratories in Korea since 1995. In 2018 as part of the program, an annual survey of cytologic data was administered to determine the current status of cytopathology practices in Korea. Methods: A questionnaire was administered to 211 cytopathology laboratories. Individual laboratories submitted their annual statistics regarding cytopathology practices, diagnoses of gynecologic samples, inadequacy rates, and gynecologic cytology-histology correlation review (CHCR) data for 2018. In addition, proficiency tests and sample adequacy assessments were conducted using five consequent gynecologic slides. Results: Over 10 million cytologic exams were performed in 2018, and this number has almost tripled since this survey was first conducted in 2004 (compounded annual growth rate of 7.2%). The number of non-gynecologic samples has increased gradually over time and comprised 24% of all exams. The overall unsatisfactory rate was 0.14%. The ratio of the cases with atypical squamous cells to squamous intraepithelial lesions accounted for up to 4.24. The major discrepancy rate of the CHCR in gynecologic samples was 0.52%. In the proficiency test, the major discrepancy rate was approximately 1%. In the sample adequacy assessment, a discrepancy was observed in 0.1% of cases. Conclusions: This study represents the current status of cytopathology practices in Korea, illustrating the importance of the Continuous Quality Improvement program for increasing the accuracy and credibility of cytopathologic exams as well as developing national cancer exam guidelines and government projects on the prevention and treatment of cancer.
Keyword
Figure
Cited by 2 articles
-
Re-Increasing Trends in Thyroid Cancer Incidence after a Short Period of Decrease in Korea: Reigniting the Debate on Ultrasound Screening
Chan Kwon Jung, Ja Seong Bae, Young Joo Park
Endocrinol Metab. 2022;37(5):816-818. doi: 10.3803/EnM.2022.1586.Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
Soon Auck Hong, Haeyoen Jung, Sung Sun Kim, Min-Sun Jin, Jung-Soo Pyo, Ji Yun Jeong, Younghee Choi, Gyungyub Gong, Yosep Chong
J Pathol Transl Med. 2022;56(6):361-369. doi: 10.4132/jptm.2022.09.21.
Reference
-
1. Lee HK, Kim SN, Khang SK, Kang CS, Yoon HK. Quality control program and its results of Korean Society for Cytopathologists. Korean J Cytopathol. 2008; 19:65–71.
Article2. Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011; 12:725–30.3. Suh M, Song S, Cho HN, et al. Trends in participation rates for the national cancer screening program in Korea, 2002-2012. Cancer Res Treat. 2017; 49:798–806.
Article4. Oh EJ, Jung CK, Kim DH, et al. Current Cytology Practices in Korea: A nationwide survey by the Korean Society for Cytopathology. J Pathol Transl Med. 2017; 51:579–87.
Article5. Jung M. National Cancer Screening Programs and evidence-based healthcare policy in South Korea. Health Policy. 2015; 119:26–32.
Article6. Nayar R, Wilbur DC. The Bethesda system for reporting cervical cytology: definitions, criteria, and explanatory notes. 3rd ed. Cham: Springer;2015.7. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017; 27:1341–6.
Article8. Lim SC, Yoo CW. Current status of and perspectives on cervical cancer screening in Korea. J Pathol Transl Med. 2019; 53:210–6.
Article9. Chong Y, Ji SJ, Kang CS, Lee EJ. Can liquid-based preparation substitute for conventional smear in thyroid fine-needle aspiration? A systematic review based on meta-analysis. Endocr Connect. 2017; 6:817–29.
Article10. Nascimento AF, Cibas ES. The ASC/SIL ratio for cytopathologists as a quality control measure: a follow-up study. Am J Clin Pathol. 2007; 128:653–6.11. Hodgson C, Cross P. Introduction and development of a digital diagnostic non gynaecological diagnostic cytology interpretative scheme. Cytopathology. 2018; 29(Suppl 1):79.12. Greaves J, Holzhauser D. Virtual cytopathology in an Austrailian EQA setting. In : 20th International Congress of Cytology; 2019 May 5-10; Sydney, Australia.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
- Current state of cytopathology residency training: a Korean national survey of pathologists
- Current Cytology Practices in Korea: A Nationwide Survey by the Korean Society for Cytopathology
- Continuous quality improvement program and its results of Korean Society for Cytopathology
- Quality Control Program and Its Results of Korean Society for Cytopathologists