Cancer Res Treat.
2020 Jul;52(3):907-916. 10.4143/crt.2019.713.
Ramosetron versus Palonosetron in Combination with Aprepitant and Dexamethasone for the Control of Highly-Emetogenic Chemotherapy-Induced Nausea and Vomiting
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul St. Mary’s Hospital, Seoul, Korea
- 2Division of Hemato-Oncology, Department of Internal Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 3Division of Hematology/Oncology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Division of Hematology/Oncology, Department of Internal Medicine, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- 5Division of Medical Oncology, Department of Internal Medicine, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 6Division of Medical Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 7Division of Hemato-Oncology, Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 8Department of Hematology-Oncology, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- 9Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea
- 10Division of Hematology & Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 11Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2504469
- DOI: http://doi.org/10.4143/crt.2019.713
Abstract
- Purpose
The purpose of this study was to compare ramosetron (RAM), aprepitant (APR), and dexamethasone (DEX) [RAD] with palonosetron (PAL), APR, and DEX [PAD] in controlling highly-emetogenic chemotherapy (HEC)–induced nausea and vomiting.
Materials and Methods
Patients were randomly assigned (1:1) to receive RAD or PAD:RAM (0.3 mg intravenously) or PAL (0.25 mg intravenously) D1, combined with APR (125 mg orally, D1 and 80 mg orally, D2-3) and DEX (12 mg orally or intravenously, D1 and 8 mg orally, D2-4). Patients were stratified by gender, cisplatin-based chemotherapy, and administration schedule. The primary endpoint was overall complete response (CR), defined as no emesis and no rescue regimen during 5 days of HEC. Secondary endpoints were overall complete protection (CP; CR+nausea score < 25 mm) and total control (TC; CR+nausea score < 5 mm). Quality of life was assessed by Functional Living Index Emesis (FLIE) questionnaire on D0 and D6.
Results
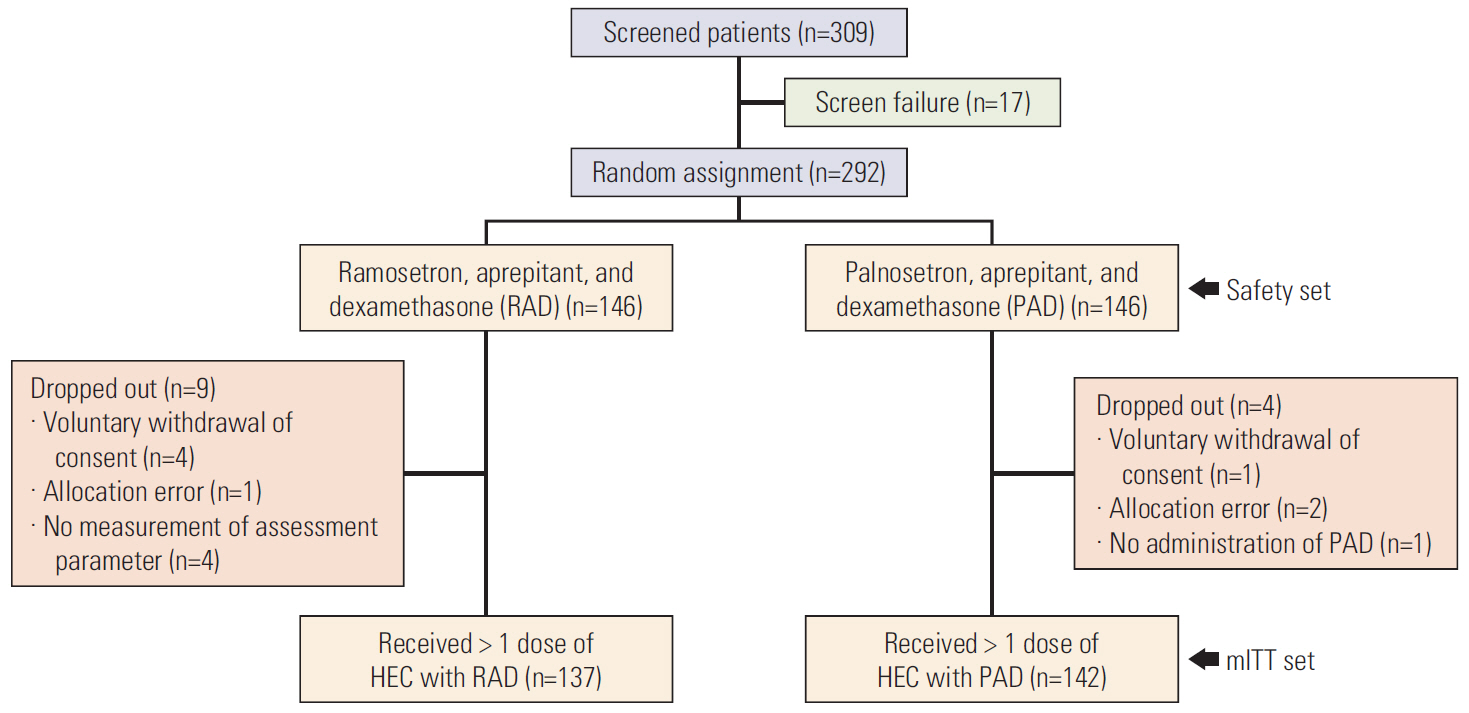
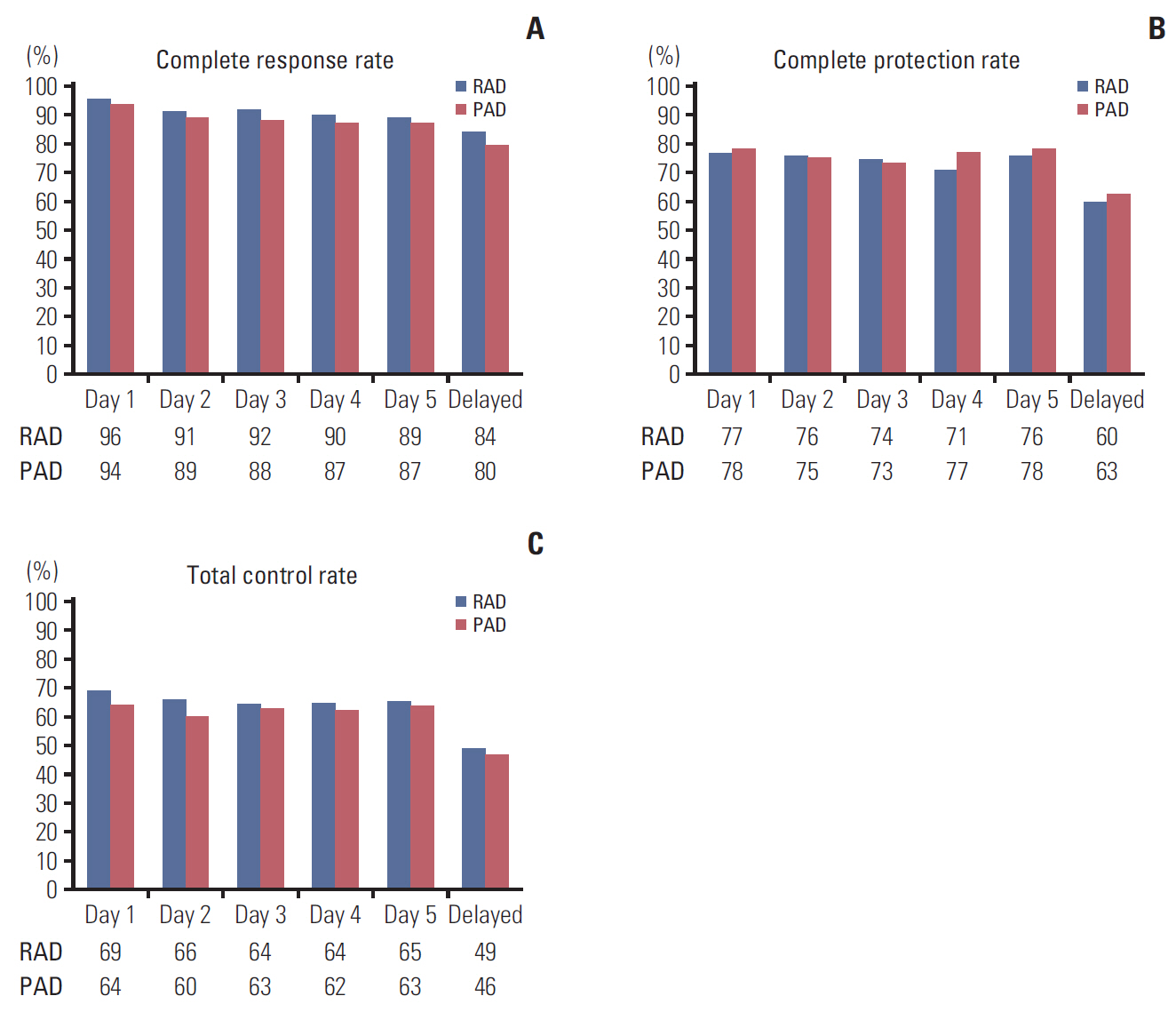
A total of 279 patients receiving RAD (n=137) or PAD (n=142) were evaluated. Overall CR rates in RAD and PAD recipients were 81.8% and 79.6% (risk difference [RD], 2.2%; 95% confidence interval [CI], −7.1 to 11.4), respectively. Overall CP and TC rates for RAD and PAD were 56.2% and 58.5% (RD, −2.3%; 95% CI, −13.9 to 9.4) and 47.5% vs. 43.7% (RD, 3.8%; 95% CI, −7.9 to 15.5), respectively. FLIE total score ≥ 108 (no impact on daily life) was comparable between RAD and PAD (73.9% vs. 73.4%, respectively). Adverse events were similar between the two groups.
Conclusion
In all aspects of efficacy, safety and QOL, RAD is non-inferior to PAD for the control of CINV in cancer patients receiving HEC.
Keyword
Figure
Reference
-
References
1. Lindley C, McCune JS, Thomason TE, Lauder D, Sauls A, Adkins S, et al. Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract. 1999; 7:59–65.
Article2. Passik SD, Kirsh KL, Rosenfeld B, McDonald MV, Theobald DE. The changeable nature of patients' fears regarding chemotherapy: implications for palliative care. J Pain Symptom Manage. 2001; 21:113–20.3. Fernandez-Ortega P, Caloto MT, Chirveches E, Marquilles R, Francisco JS, Quesada A, et al. Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients' quality of life. Support Care Cancer. 2012; 20:3141–8.
Article4. Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003; 21:4112–9.
Article5. Grunberg SM, Koeller JM. Palonosetron: a unique 5-HT3-receptor antagonist for the prevention of chemotherapy-induced emesis. Expert Opin Pharmacother. 2003; 4:2297–303.
Article6. Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003; 98:2473–82.7. Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003; 14:1570–7.
Article8. National Comprehensive Cancer Network. Antiemesis (version 1.2020) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2020. [cited 2020 Feb 27]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.9. Rabasseda X. Ramosetron, a 5-HT3 receptor antagonist for the control of nausea and vomiting. Drugs Today (Barc). 2002; 38:75–89.
Article10. Kim JS, Kim JY, Lee SJ, Park DK, Namgung H, Kim CN, et al. Multicenter nonrandomized trial of ramosetron versus palonosetron in controlling chemotherapy-induced nausea and vomiting for colorectal cancer. Ann Surg Treat Res. 2014; 87:9–13.
Article11. Martin AR, Pearson JD, Cai B, Elmer M, Horgan K, Lindley C. Assessing the impact of chemotherapy-induced nausea and vomiting on patients' daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Support Care Cancer. 2003; 11:522–7.12. Rhodes VA. Criteria for assessment of nausea, vomiting, and retching. Oncol Nurs Forum. 1997; 24(7 Suppl):13–9.13. . National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) [Internet]. Bethesda, MD: National Cancer Institute;2010. [cited 2019 Sep 11]. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.14. Suzuki K, Yamanaka T, Hashimoto H, Shimada Y, Arata K, Matsui R, et al. Randomized, double-blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol. 2016; 27:1601–6.
Article15. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011; 9:188–95.
Article16. Hesketh PJ, Rossi G, Rizzi G, Palmas M, Alyasova A, Bondarenko I, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014; 25:1340–6.
Article17. Roila F, Ruggeri B, Ballatori E, Fatigoni S, Caserta C, Licitra L, et al. Aprepitant versus metoclopramide, both combined with dexamethasone, for the prevention of cisplatin-induced delayed emesis: a randomized, double-blind study. Ann Oncol. 2015; 26:1248–53.
Article18. Gralla RJ, Bosnjak SM, Hontsa A, Balser C, Rizzi G, Rossi G, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014; 25:1333–9.
Article19. Wu F, Lin X, Yang Z, Sun Z, Zeng F, Heng J, et al. Phase III randomized trial of palonosetron and dexamethasone with or without aprepitant to prevent nausea and vomiting induced by full-dose single-day cisplatin-based chemotherapy in lung cancer. Clin Lung Cancer. 2018; 19:e913–8.
Article20. Zhang L, Lu S, Feng J, Dechaphunkul A, Chang J, Wang D, et al. A randomized phase III study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Ann Oncol. 2018; 29:452–8.
Article21. Kim HJ, Shin SW, Song EK, Lee NR, Kim JS, Ahn JS, et al. Ramosetron versus ondansetron in combination with aprepitant and dexamethasone for the prevention of highly emetogenic chemotherapy-induced nausea and vomiting: a multicenter, randomized phase III trial, KCSG PC10-21. Oncologist. 2015; 20:1440–7.
Article22. Hirata T, Keto Y, Funatsu T, Akuzawa S, Sasamata M. Evaluation of the pharmacological profile of ramosetron, a novel therapeutic agent for irritable bowel syndrome. J Pharmacol Sci. 2007; 104:263–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative study of an aprepitant regimen with an ondansetron regimen, for efficacy in gynecological cancer patients with chemotherapy
- A Phase II Study to Evaluate the Efficacy of Ramosetron, Aprepitant, and Dexamethasone in Preventing Cisplatin-Induced Nausea and Vomiting in Chemotherapy-Naive Cancer Patients
- Palonosetron versus granisetron in combination with aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with gynecologic cancer
- Multicenter nonrandomized trial of ramosetron versus palonosetron in controlling chemotherapy-induced nausea and vomiting for colorectal cancer
- Comparative Study of an Ondansetron and a Ramosetron an Aprepitant in the Control of Nausea and Vomiting in Gynecologinc Cancer Patient with Chemotherapy