Kosin Med J.
2020 Jun;35(1):26-37. 10.7180/kmj.2020.35.1.26.
Influence of Cold Ischemia Time and Storage Period on DNA Quality and Biomarker Research in Biobanked Colorectal Cancer Tissues
- Affiliations
-
- 1Department of Pathology, Dong-A University College of Medicine, Busan, Korea
- KMID: 2503726
- DOI: http://doi.org/10.7180/kmj.2020.35.1.26
Abstract
Objectives
Biobanking plays an important role in future research. Assessment and control of the preanalytical variables of biobanked tissues are fundamentals for the optimal use of biospecimens.
Methods
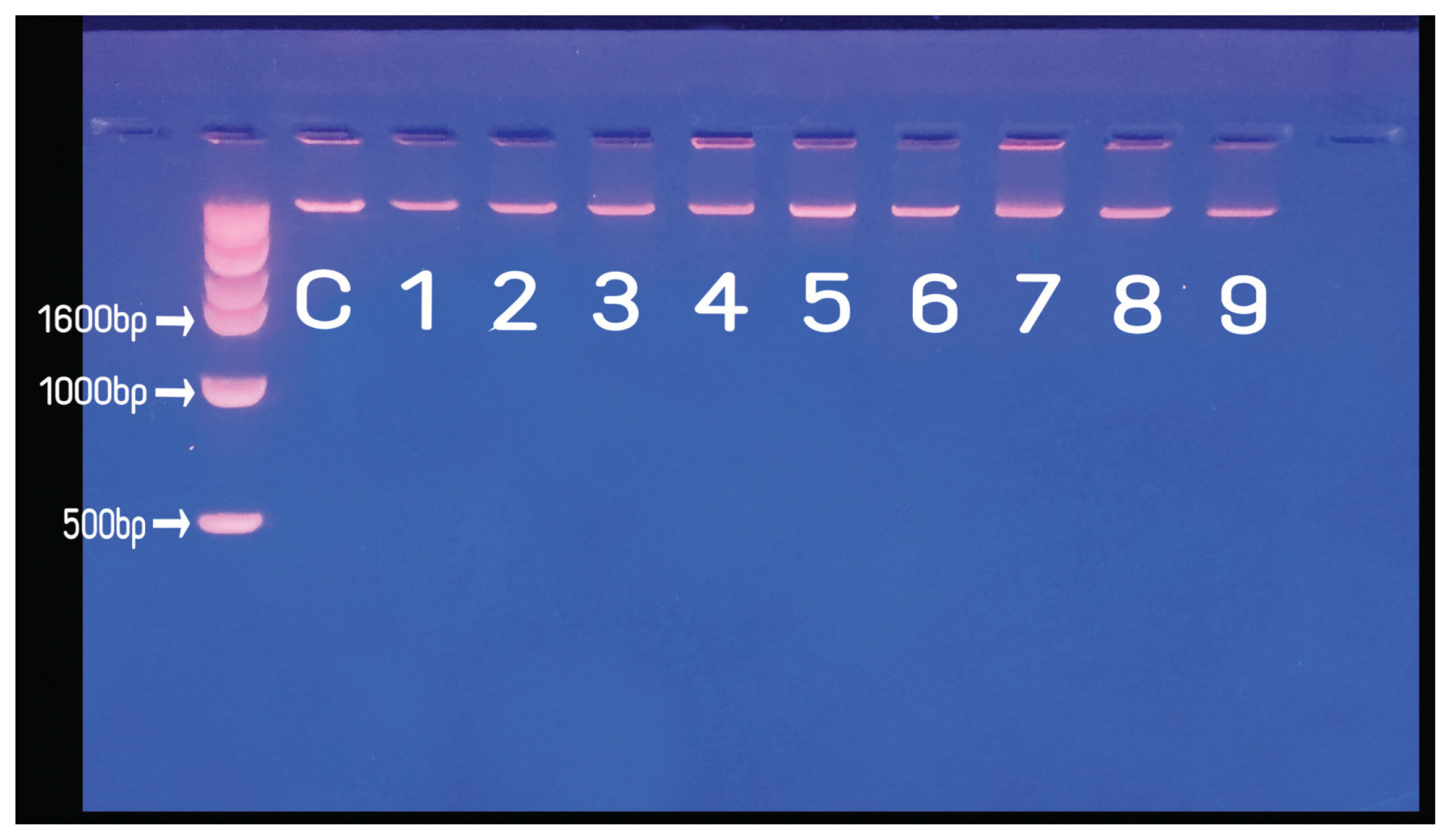
Forty-five colorectal cancer (CRC) tissues stored at -80℃in Bio-Resource Bank were evaluated to define the influence of cold ischemia time (CIT) and storage period (SP) on DNA quality in biobanked tissues. Three CITs (less than 30 minutes (CIT-1), 30-45 minutes (CIT-2), and 45-60 minutes (CIT-3)) and three SPs (less than 1 year (SP-1), 2-3 years (SP-2), and 4-5 years (SP-3)) were chosen. NanoDrop spectrophotometer was used to determine the 260/280 ratio for DNA purity. DNA integrity was analyzed by a UV transilluminator following electrophoresis on 2% agarose gel. To evaluate the practical usability of DNA for biomarker research, KRAS mutation status was assessed by PCR amplification.
Results
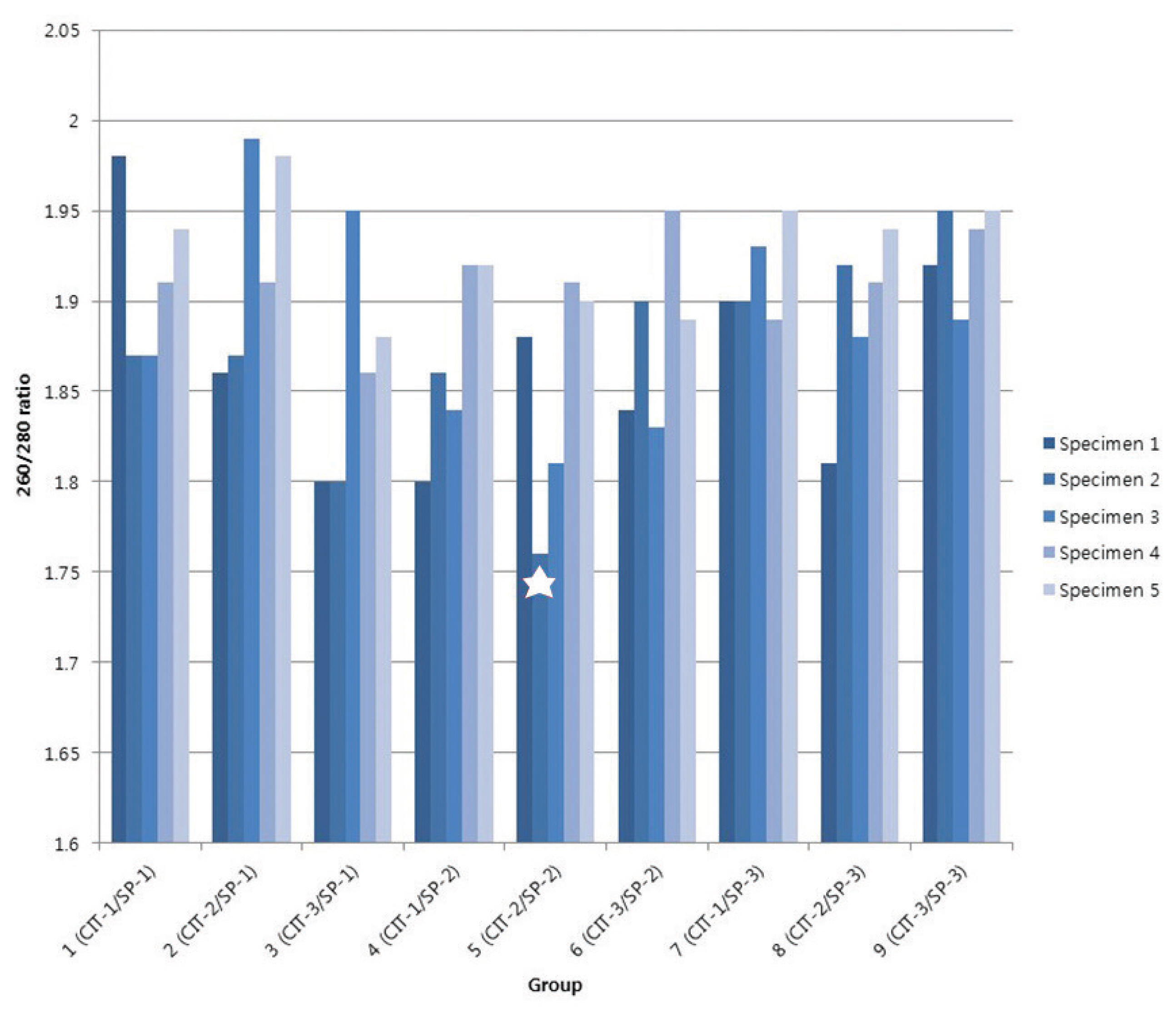
All DNA specimens had a 260/280 ratio ranging between 1.8 and 2.0 with the exception of one specimen (CIT- 2/SP-2 group). For DNA integrity, DNA appeared as a compact, high-molecular-weight band with no or scanty low-molecular- weight smears. The concordance of KRAS mutation status between paired biobanked frozen tissues and formalin-fixed paraffin-embedded tissues was 100%. DNA remained stable in CRC tissues kept at room temperature for up to 1 hour and long-term storage up to 5 years.
Conclusions
Storage conditions of our biobank are suitable for long-term (at least five years) specimen preservation with high DNA quality. These results have practical implications that could affect banking guidelines.
Keyword
Figure
Reference
-
1. Tang W, Hu Z, Muallem H, Gulley ML. Quality assurance of RNA expression profiling in clinical laboratories. J Mol Diagn. 2012; 14:1–11.
Article2. Watson RW, Kay EW, Smith D. Integrating biobanks: addressing the practical and ethical issues to deliver a valuable tool for cancer research. Nat Rev Cancer. 2010; 10:646–51.
Article3. Ellervik C, Vaught J. Preanalytical variables affecting the integrity of human biospecimens in biobanking. Clin Chem. 2015; 61:914–34.
Article4. Qualman SJ, France M, Grizzle WE, LiVolsi VA, Moskaluk CA, Ramirez NC, et al. Establishing a tumour bank: banking, informatics and ethics. Br J Cancer. 2004; 90:1115–9.
Article5. Chu TY, Hwang KS, Yu MH, Lee HS, Lai HC, Liu JY. A research-based tumor tissue bank of gynecologic oncology: characteristics of nucleic acids extracted from normal and tumor tissues from different sites. Int J Gynecol Cancer. 2002; 12:171–6.
Article6. Shabihkhani M, Lucey GM, Wei B, Mareninov S, Lou JJ, Vinters HV, et al. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin Biochem. 2014; 47:258–66.
Article7. Spruessel A, Steimann G, Jung M, Lee SA, Carr T, Fentz AK, et al. Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. Biotechniques. 2004; 36:1030–7.
Article8. Kong F, Zhang W, Qiao L, Li Q, Li H, Cao J, et al. Establishment and quality evaluation of a glioma biobank in Beijing Tiantan Hospital. Peer J. 2018; 6:e4450.9. Grizzle WE, Otali D, Sexton KC, Atherton DS. Effects of cold ischemia on gene expression: a review and commentary. Biopreserv Biobank. 2016; 14:548–58.
Article10. Kelly R, Albert M, de Ladurantaye M, Moore M, Dokun O, Bartlett JMS. RNA and DNA integrity remain stable in frozen tissue after long-term storage at cryogenic temperatures: a report from the Ontario tumour bank. Biopreserv Biobank. 2019; 17:282–7.
Article11. Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017; 170:17–33.
Article12. Glasel JA. Validity of nucleic acid purities monitored by 260nm/280nm absorbance ratios. Biotechniques. 1995; 18:62–3.13. Jewell SD, Srinivasan M, McCart LM, Williams N, Grizzle WH, LiVolsi V, et al. Analysis of the molecular quality of human tissues: an experience from the Cooperative Human Tissue Network. Am J Clin Pathol. 2002; 118:733–41.14. Lalmahomed ZS, Coebergh van den Braak RRJ, Oomen MHA, Arshad SP, Riegman PHJ, IJzermans JNM, et al. Multicenter fresh frozen tissue sampling in colorectal cancer: does the quality meet the standards for state of the art biomarker research? Cell Tissue Bank. 2017; 18:425–31.
Article15. Musella V, Verderio P, Reid JF, Pizzamiglio S, Gariboldi M, Callari M, et al. Effects of warm ischemic time on gene expression profiling in colorectal cancer tissues and normal mucosa. PLoS One. 2013; 8:e53406.
Article16. Bao WG, Zhang X, Zhang JG, Zhou WJ, Bi TN, Wang JC, et al. Biobanking of fresh-frozen human colon tissues: impact of tissue ex-vivo ischemia times and storage periods on RNA quality. Ann Surg Oncol. 2013; 20(17):37–44.
Article17. Guerrera F, Tabbò F, Bessone L, Maletta F, Gaudiano M, Ercole E, et al. The influence of tissue ischemia time on RNA integrity and patient-derived xenografts (PDX) engraftment rate in a non-small cell lung cancer (NSCLC) biobank. PLoS One. 2016; 11:e014 5100.
Article18. Tsilimigras DI, Ntanasis-Stathopoulos I, Bagante F, Moris D, Cloyd J, Spartalis E, et al. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: a systematic review of the current evidence. Surg Oncol. 2018; 27:280–8.19. Pennock ND, Jindal S, Horton W, Sun D, Narasimhan J, Carbone L, et al. RNA-seq from archival FFPE breast cancer samples: molecular pathway fidelity and novel discovery. BMC Med Genomics. 2019; 12:195.
Article20. Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002; 161:1961–71.
Article21. Hedegaard J, Thorsen K, Lund MK, Hein AM, Hamilton-Dutoit SJ, Vang S, et al. Next-generation sequencing of RNA and DNA isolated from paired fresh-frozen and formalin-fixed paraffin-embedded samples of human cancer and normal tissue. PLoS One. 2014; 9:e98187.
Article22. Bins S, Cirkel GA, Gadellaa-Van Hooijdonk CG, Weeber F, Numan IJ, Bruggink AH, et al. Implementation of a multicenter biobanking collaboration for Next-generation sequencing-based biomarker discovery based on fresh frozen pretreatment tumor tissue biopsies. Oncologist. 2017; 22:33–40.
Article23. von Ahlfen S, Missel A, Bendrat K, Schlumpberger M. Determinants of RNA quality from FFPE samples. PLoS One. 2007; 2:e1261.
Article24. Hojat A, Wei B, Olson MG, Mao Q, Yong WH. Procurement and storage of surgical biospecimens. Methods Mol Biol. 2019; 1897:65–76.
Article25. Choi C. Development of standard operation manual for national biobank of Korea. Report No. 2008-E00353-00. Seoul: Korea Centers for Disease Control and Prevention;2008.26. Park SY, Baek HA, Kwa HJ, Hong SH, Park HS, Jang KY, et al. Quality control program for fresh frozen tissue and its results of Chonbuk National University Hospital National Biobank of Korea. Korean J Pathol. 2010; 44:295–301.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Delay in the Snap Freezing of Colorectal Cancer Tissues on the Quality of DNA and RNA
- Mutations of DNA repair associated gene, APEX in human colorectal cancer
- Stool DNA Testing for Colorectal Cancer: Development and Advances
- In silico Identification of SFRP1 as a Hypermethylated Gene in Colorectal Cancers
- Prognostic significance of DNA ploidy in colorectal cancer