Ann Pediatr Endocrinol Metab.
2020 Jun;25(2):126-131. 10.6065/apem.1938144.072.
Effects of long-term growth hormone therapy in a girl with Floating-Harbor syndrome
- Affiliations
-
- 11 Department of Pediatrics, Inje University Busan Paik Hospital, Busan, Korea
- 2Department of Diagnostic Laboratory Medicine, Inje University Busan Paik Hospital, Busan, Korea
- 3Rare Genetic Disease Research Center, 3 Billion, Seoul, Korea
- KMID: 2503364
- DOI: http://doi.org/10.6065/apem.1938144.072
Abstract
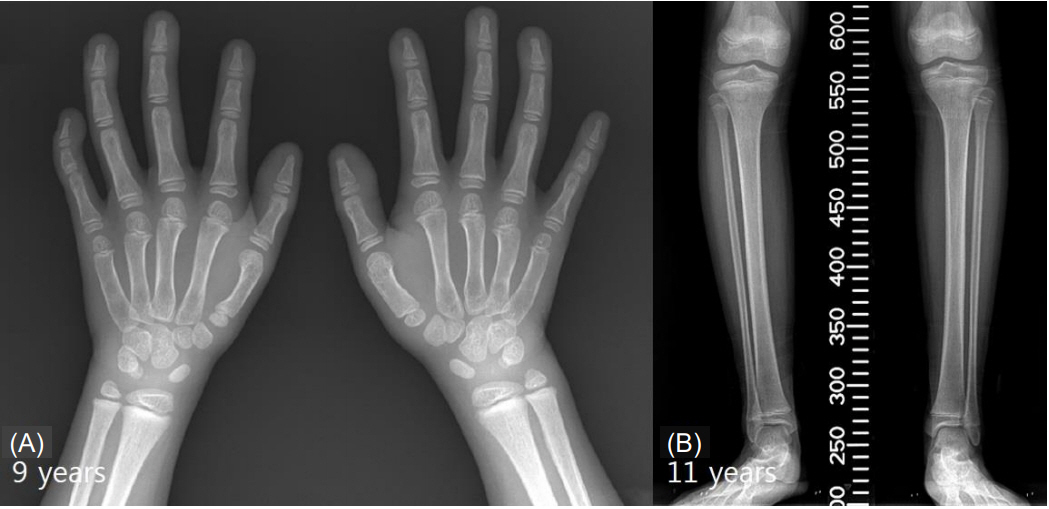
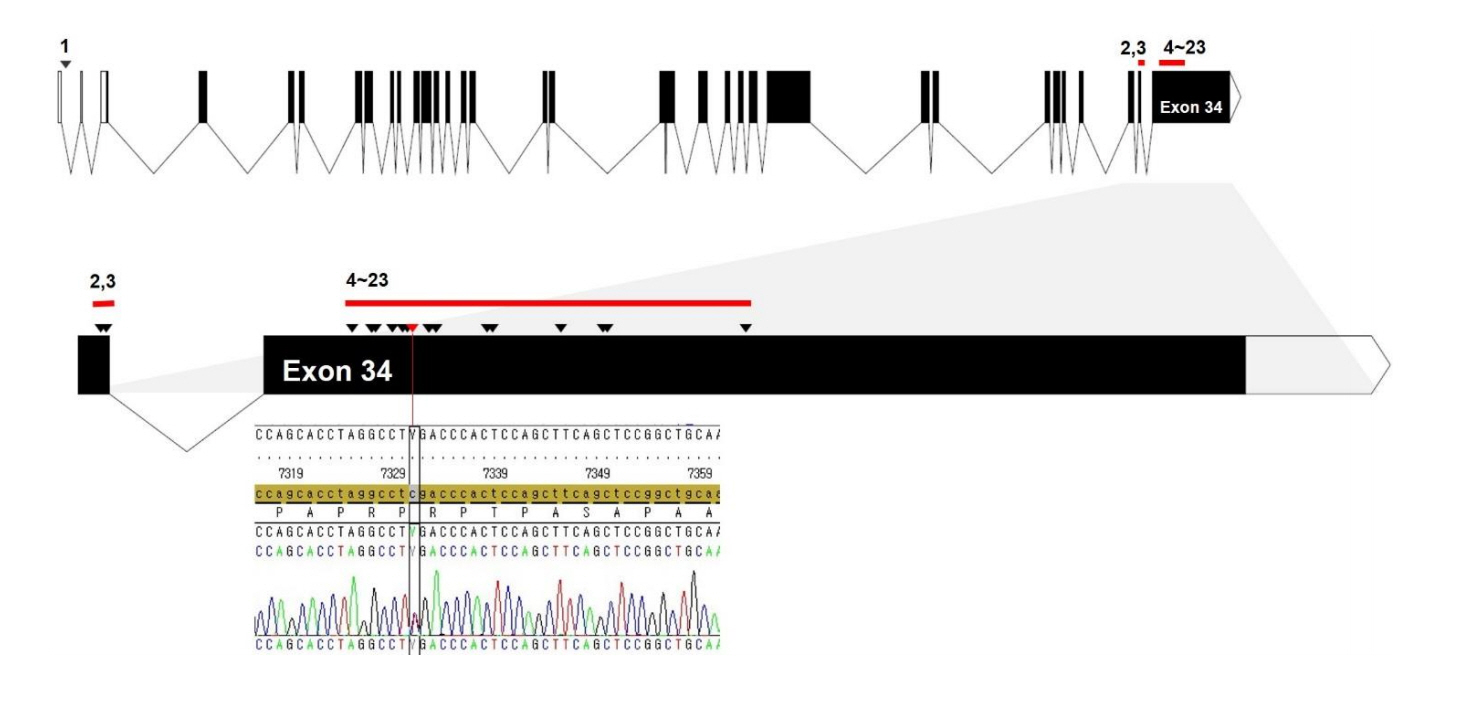
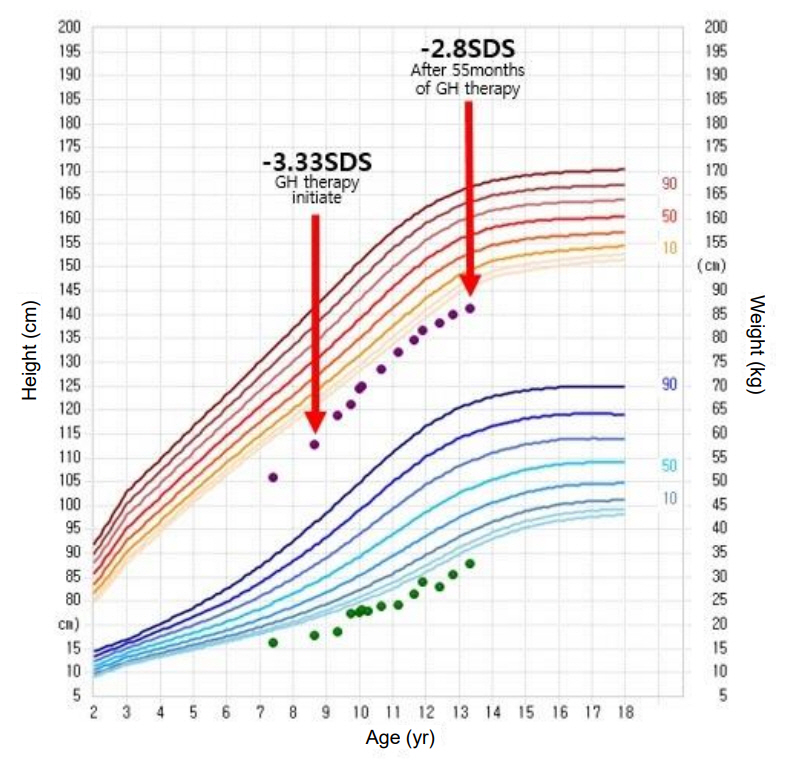
- Floating-Harbor syndrome is a rare autosomal dominant disorder that presents with short stature, facial dysmorphism, significantly delayed bone age, skeletal abnormalities, speech and language problems, and intellectual disabilities. Although short stature is one of the main clinical manifestations, use of growth hormone therapy in Floating-Harbor syndrome patients has been limited. Only a few reports have investigated the response to growth hormone therapy with regard to final adult height. We report the case of a 7-year-old girl with FloatingHarbor syndrome and a heterozygous mutation, c.7330C > T (p.Arg2444*), in the SRCAP gene. The patient exhibited dysmorphic facial features, severe intellectual disabilities, obsessive-compulsive and aggressive behaviors, and short stature without growth hormone deficiency. Her height standard deviation score improved after 55 months of growth hormone therapy.
Keyword
Figure
Cited by 1 articles
-
Empty Sella Syndrome Associated with Growth Hormone Deficiency: the First Case Report of Weiss-Kruszka Syndrome
Jisun Park, Dong Jun Ha, Go Hun Seo, Seri Maeng, Sung Mo Kang, Sujin Kim, Ji Eun Lee
J Korean Med Sci. 2021;36(18):e133. doi: 10.3346/jkms.2021.36.e133.
Reference
-
References
1. Hood RL, Schenkel LC, Nikkel SM, Ainsworth PJ, Pare G, Boycott KM, et al. The defining DNA methylation signature of Floating-Harbor Syndrome. Sci Rep. 2016; 6:38803.
Article2. Hood RL, Lines MA, Nikkel SM, Schwartzentruber J, Beaulieu C, Nowaczyk MJ, et al. Mutations in SRCAP, encoding SNF2-related CREBBP activator protein, cause Floating-Harbor syndrome. Am J Hum Genet. 2012; 90:308–13.
Article3. Leisti J, Hollister DW, Rimoin DL. The Floating-Harbor syndrome. Birth Defects Orig Artic Ser. 1975; 11:305.4. Nagasaki K, Asami T, Sato H, Ogawa Y, Kikuchi T, Saitoh A, et al. Long-term follow-up study for a patient with FloatingHarbor syndrome due to a hotspot SRCAP mutation. Am J Med Genet A. 2014; 164A:731–5.5. Nikkel SM, Dauber A, de Munnik S, Connolly M, Hood RL, Caluseriu O, et al. The phenotype of Floating-Harbor syndrome: clinical characterization of 52 individuals with mutations in exon 34 of SRCAP. Orphanet J Rare Dis. 2013; 8:63.
Article6. White SM, Morgan A, Da Costa A, Lacombe D, Knight SJ, Houlston R, et al. The phenotype of Floating-Harbor syndrome in 10 patients. Am J Med Genet A. 2010; 152A:821–9.
Article7. Arpin S, Afenjar A, Dubern B, Toutain A, Cabrol S, Héron D. Floating-Harbor Syndrome: report on a case in a mother and daughter, further evidence of autosomal dominant inheritance. Clin Dysmorphol. 2012; 21:11–4.8. Galli-Tsinopoulou A, Kyrgios I, Emmanouilidou E, Maggana I, Kotanidou E, Kokka P, et al. Growth hormone deficiency: an unusual presentation of floating harbor syndrome. Hormones (Athens). 2011; 10:236–40.
Article9. Seo GH, Park JY, Kim SH, Lee JS, Oh Arum, Lee Y, et al. High diagnostic yield and clinical utility of WES for patients with undiagnosed genetic disorder by automating variant interpretation. BioRxiv. 2019; May. 9. https://doi.org/10.1101/628438.
Article10. Johnston H, Kneer J, Chackalaparampil I, Yaciuk P, Chrivia J. Identification of a novel SNF2/SWI2 protein family member, SRCAP, which interacts with CREB-binding protein. J Biol Chem. 1999; 274:16370–6.
Article11. Budisteanu M, Bögershausen N, Papuc SM, Moosa S, Thoenes M, Riga D, et al. Floating-Harbor syndrome: presentation of the first Romanian patient with a SRCAP mutation and review of the literature. Balkan J Med Genet. 2018; 21:83–6.12. Choi EM, Lee DH, Kang SJ, Shim YJ, Kim HS, Kim JS, et al. The first Korean case with Floating-Harbor syndrome with a novel SRCAP mutation diagnosed by targeted exome sequencing. Korean J Pediatr. 2018; 61:403–6.
Article13. García RJ, Kant SG, Wit JM, Mericq V. Clinical and genetic characteristics and effects of long-term growth hormone therapy in a girl with Floating-Harbor syndrome. J Pediatr Endocrinol Metab. 2012; 25:207–12.
Article14. Zhang S, Chen S, Qin H, Yuan H, Pi Y, Yang Y, et al. Novel genotypes and phenotypes among Chinese patients with Floating-Harbor syndrome. Orphanet J Rare Dis. 2019; 14:144.
Article15. Alfares A, Alfadhel M, Wani T, Alsahli S, Alluhaydan I, Al Mutairi F, et al. A multicenter clinical exome study in unselected cohorts from a consanguineous population of Saudi Arabia demonstrated a high diagnostic yield. Mol Genet Metab. 2017; 121:91–5.
Article16. Seifert W, Meinecke P, Krüger G, Rossier E, Heinritz W, Wüsthof A, et al. Expanded spectrum of exon 33 and 34 mutations in SRCAP and follow-up in patients with Floating-Harbor syndrome. BMC Med Genet. 2014; 15:127.
Article17. Kehrer M, Beckmann A, Wyduba J, Finckh U, Dufke A, Gaiser U, et al. Floating-Harbor syndrome: SRCAP mutations are not restricted to exon 34. Clin Genet. 2014; 85:498–9.18. Le Goff C, Mahaut C, Bottani A, Doray B, Goldenberg A, Moncla A, et al. Not all floating-harbor syndrome cases are due to mutations in exon 34 of SRCAP. Hum Mutat. 2013; 34:88–92.19. Milani D, Scuvera G, Gatti M, Tolva G, Bonarrigo F, Esposito S, et al. Perthes disease: a new finding in FloatingHarbor syndrome. Am J Med Genet A. 2018; 176:703–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prader-Willi syndrome and growth hormone therapy: exploring the precise management of hypothalamic short stature: A review
- First Turkish Patient with Floating Harbor Syndrome with Additional Findings: Cryptorchidim and Microcephaly
- Long-term Growth Response to Growth Hormone Therapy in Children with Growth Hormone Deficiency
- Effect of yeast-derived methionyl recombinant growth hormone on growth hormone deficient dwarf
- Growth Hormone Therapy in Short Stature Children