Korean J Physiol Pharmacol.
2020 May;24(3):233-240. 10.4196/kjpp.2020.24.3.233.
ATG5 knockout promotes paclitaxel sensitivity in drug-resistant cells via induction of necrotic cell death
- Affiliations
-
- 1Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University
- 2INU Human Genome Research Center, Incheon National University, Incheon 22012, Korea
- KMID: 2502505
- DOI: http://doi.org/10.4196/kjpp.2020.24.3.233
Abstract
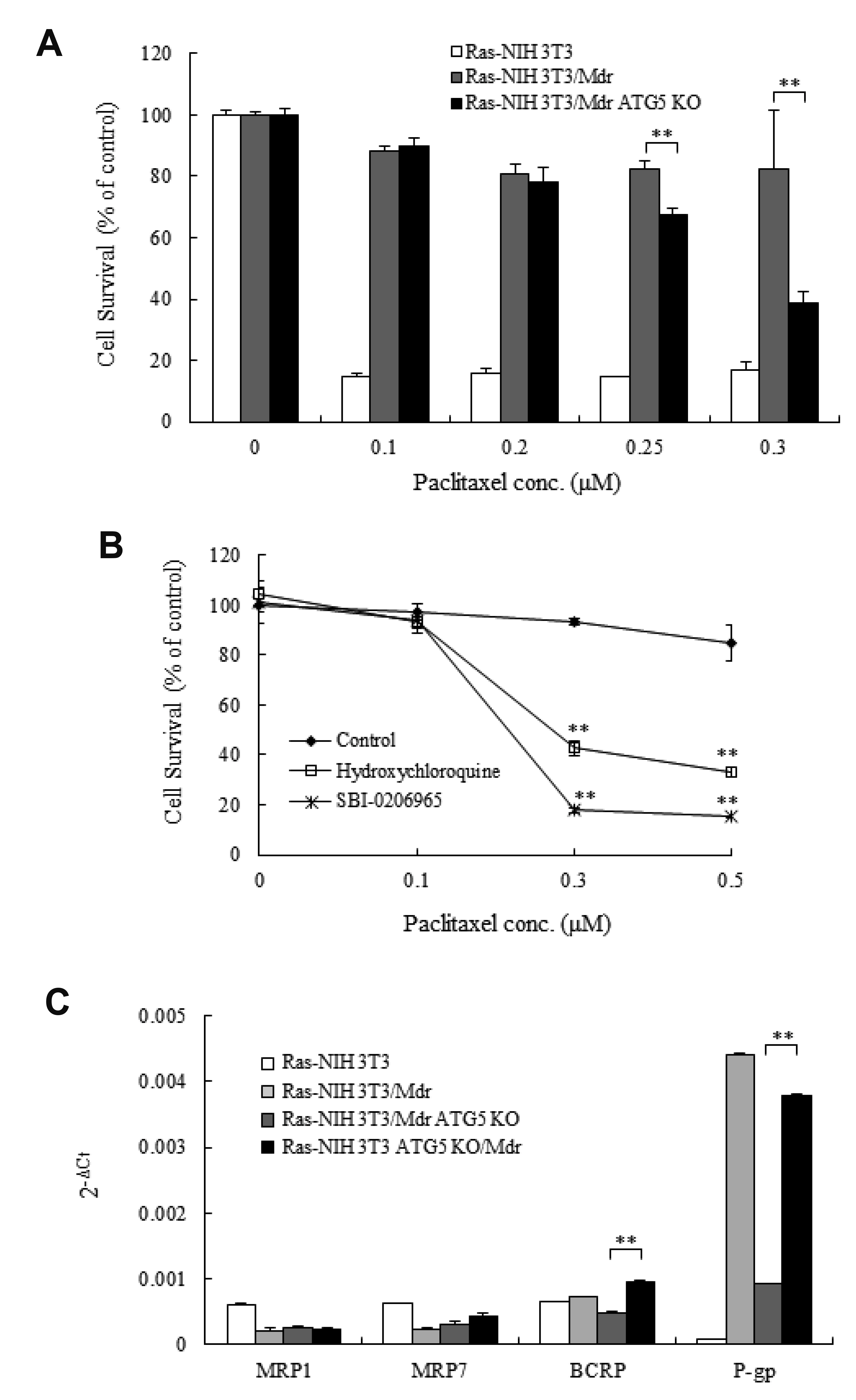
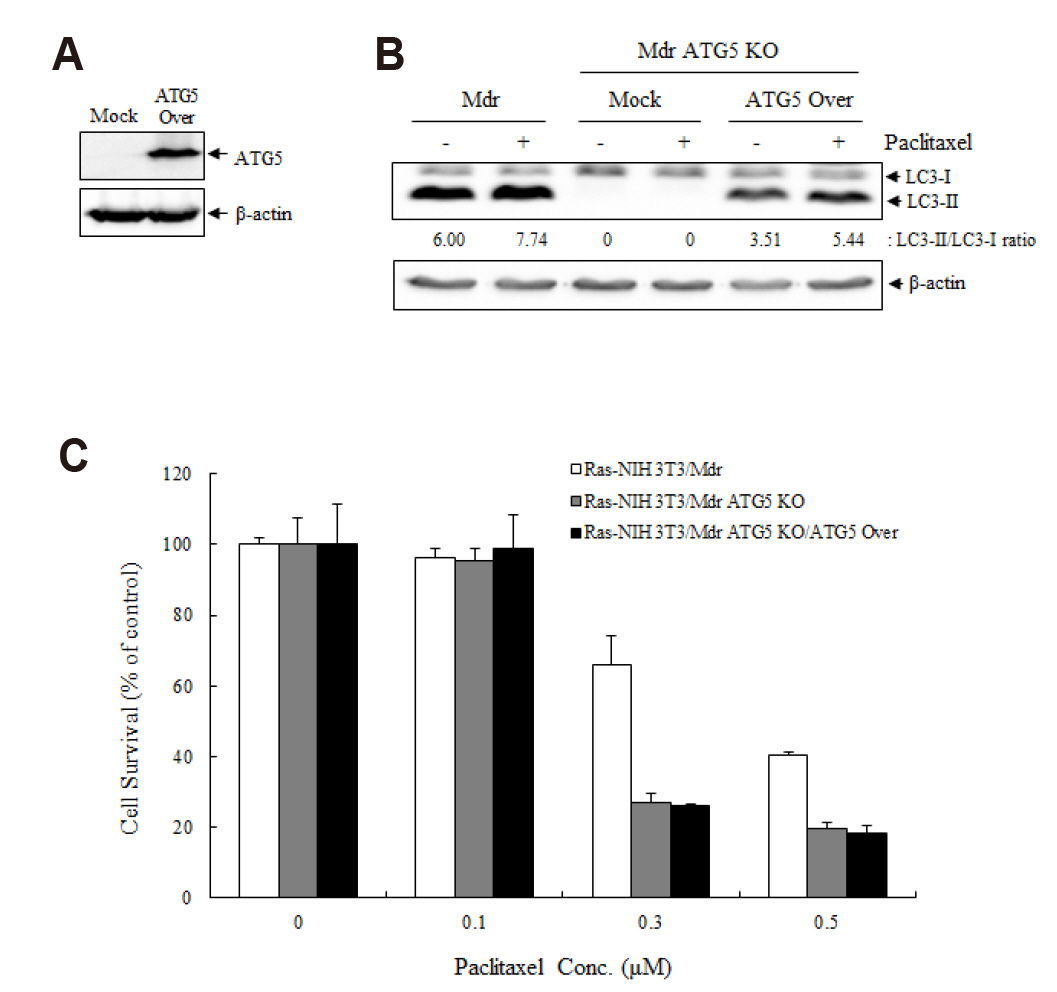
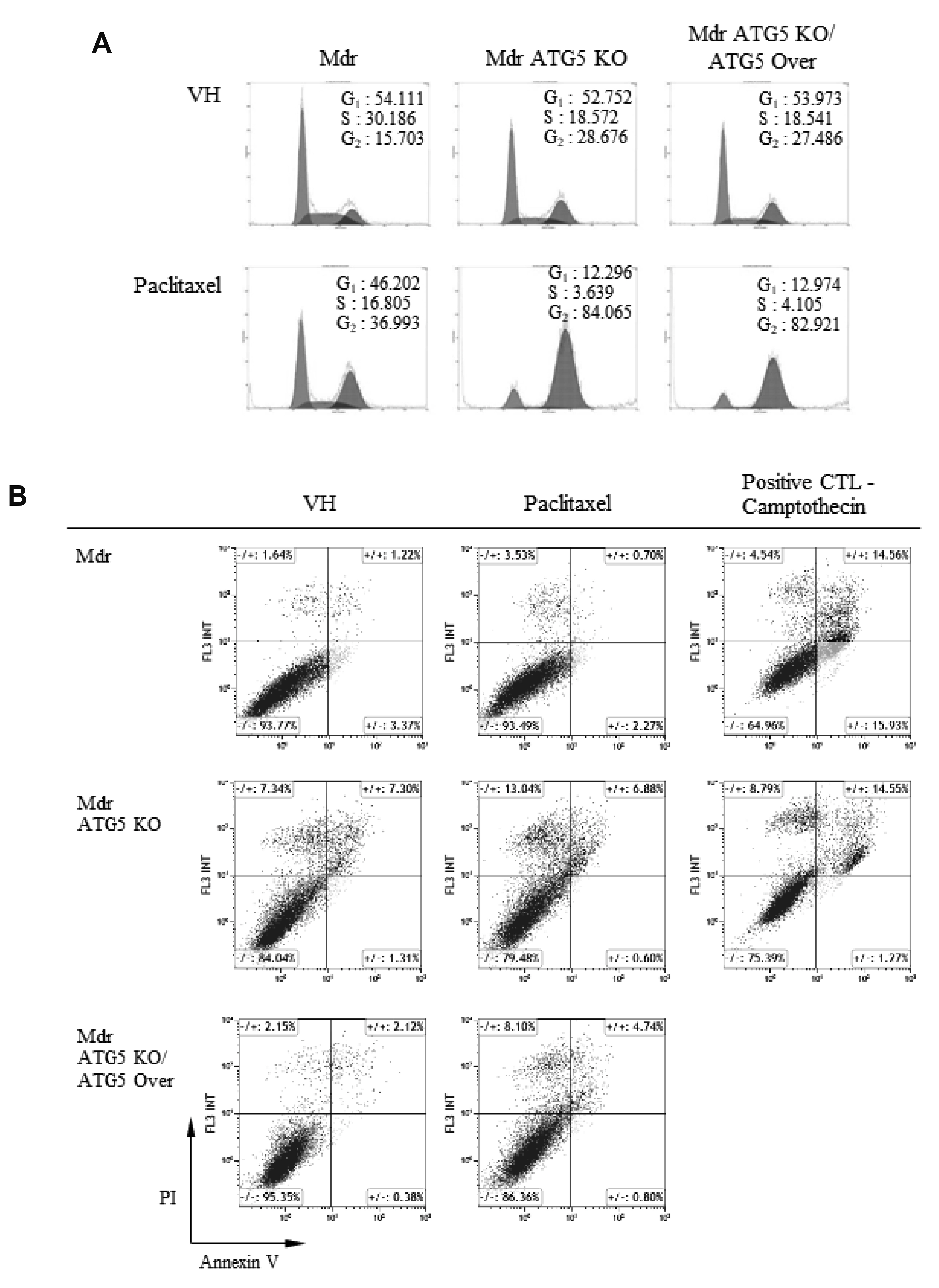
- Autophagy regulators are often effective as potential cancer therapeutic agents. Here, we investigated paclitaxel sensitivity in cells with knockout (KO) of ATG5 gene. The ATG5 KO in multidrug resistant v-Ha-ras -transformed NIH 3T3 cells (Ras-NIH 3T3/Mdr) was generated using the CRISPR/Cas9 technology. The qPCR and LC3 immunoblot confirmed knockout of the gene and protein of ATG5, respectively. The ATG5 KO restored the sensitivity of Ras-NIH 3T3/Mdr cells to paclitaxel. Interestingly, ATG5 overexpression restored autophagy function in ATG5 KO cells, but failed to rescue paclitaxel resistance. These results raise the possibility that low level of resistance to paclitaxel in ATG5 KO cells may be related to other roles of ATG5 independent of its function in autophagy. The ATG5 KO significantly induced a G2/M arrest in cell cycle progression. Additionally, ATG5 KO caused necrosis of a high proportion of cells after paclitaxel treatment. These data suggest that the difference in sensitivity to paclitaxel between ATG5 KO and their parental MDR cells may result from the disparity in the proportions of necrotic cells in both populations. Thus, our results demonstrate that the ATG5 KO in paclitaxel resistant cells leads to a marked G2/M arrest and sensitizes cells to paclitaxel-induced necrosis.
Keyword
Figure
Reference
-
1. Mizushima N, Komatsu M. 2011; Autophagy: renovation of cells and tissues. Cell. 147:728–741. DOI: 10.1016/j.cell.2011.10.026. PMID: 22078875.
Article2. Saha S, Panigrahi DP, Patil S, Bhutia SK. 2018; Autophagy in health and disease: a comprehensive review. Biomed Pharmacother. 104:485–495. DOI: 10.1016/j.biopha.2018.05.007. PMID: 29800913.
Article3. Singh SS, Vats S, Chia AY, Tan TZ, Deng S, Ong MS, Arfuso F, Yap CT, Goh BC, Sethi G, Huang RY, Shen HM, Manjithaya R, Kumar AP. 2018; Dual role of autophagy in hallmarks of cancer. Oncogene. 37:1142–1158. DOI: 10.1038/s41388-017-0046-6. PMID: 29255248.
Article4. White E. 2012; Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 12:401–410. DOI: 10.1038/nrc3262. PMID: 22534666. PMCID: PMC3664381.
Article5. Veldhoen RA, Banman SL, Hemmerling DR, Odsen R, Simmen T, Simmonds AJ, Underhill DA, Goping IS. 2013; The chemotherapeutic agent paclitaxel inhibits autophagy through two distinct mechanisms that regulate apoptosis. Oncogene. 32:736–746. DOI: 10.1038/onc.2012.92. PMID: 22430212.
Article6. Liu EY, Ryan KM. 2012; Autophagy and cancer--issues we need to digest. J Cell Sci. 125(Pt 10):2349–2358. DOI: 10.1242/jcs.093708. PMID: 22641689.7. Ahn JH, Lee YW, Ahn SK, Lee M. 2014; Oncogenic BRAF inhibitor UAI-201 induces cell cycle arrest and autophagy in BRAF mutant glioma cells. Life Sci. 104:38–46. DOI: 10.1016/j.lfs.2014.03.026. PMID: 24721513.
Article8. Kim NY, Lee M. 2014; Autophagy-mediated growth inhibition of malignant glioma cells by the BH3-mimetic gossypol. Mol Cell Toxicol. 10:157–164. DOI: 10.1007/s13273-014-0017-8.
Article9. Kim NY, Han BI, Lee M. 2016; Cytoprotective role of autophagy against BH3 mimetic gossypol in ATG5 knockout cells generated by CRISPR-Cas9 endonuclease. Cancer Lett. 370:19–26. DOI: 10.1016/j.canlet.2015.10.008. PMID: 26476415.
Article10. Zhou J, Giannakakou P. 2005; Targeting microtubules for cancer chemotherapy. Curr Med Chem Anticancer Agents. 5:65–71. DOI: 10.2174/1568011053352569. PMID: 15720262.
Article11. Weaver BA. 2014; How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 25:2677–2681. DOI: 10.1091/mbc.e14-04-0916. PMID: 25213191. PMCID: PMC4161504.
Article12. McGrogan BT, Gilmartin B, Carney DN, McCann A. 2008; Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 1785:96–132. DOI: 10.1016/j.bbcan.2007.10.004. PMID: 18068131.
Article13. Gottesman MM, Fojo T, Bates SE. 2002; Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2:48–58. DOI: 10.1038/nrc706. PMID: 11902585.
Article14. Horwitz SB, Cohen D, Rao S, Ringel I, Shen HJ, Yang CP. 1993; Taxol: mechanisms of action and resistance. J Natl Cancer Inst Monogr. (15):55–61. PMID: 7912530.15. Liu Z, Zhu G, Getzenberg RH, Veltri RW. 2015; The upregulation of PI3K/Akt and MAP kinase pathways is associated with resistance of microtubule-targeting drugs in prostate cancer. J Cell Biochem. 116:1341–1349. DOI: 10.1002/jcb.25091. PMID: 25640606.
Article16. Ding J, Li M, Deng L, Li T. 2018; Study on biological characteristics and mechanism of paclitaxel induced drug resistance in endometrial carcinoma cells. Biomed Res Int. 2018:8372085. DOI: 10.1155/2018/8372085. PMID: 30175145. PMCID: PMC6098927.
Article17. Tan M, Jing T, Lan KH, Neal CL, Li P, Lee S, Fang D, Nagata Y, Liu J, Arlinghaus R, Hung MC, Yu D. 2002; Phosphorylation on tyrosine-15 of p34(Cdc2) by ErbB2 inhibits p34(Cdc2) activation and is involved in resistance to taxol-induced apoptosis. Mol Cell. 9:993–1004. DOI: 10.1016/S1097-2765(02)00510-5. PMID: 12049736.
Article18. Kwon WS, Rha SY, Jeung HC, Kim TS, Chung HC. 2017; Modulation of HAT activity by the BRCA2 N372H variation is a novel mechanism of paclitaxel resistance in breast cancer cell lines. Biochem Pharmacol. 138:163–173. DOI: 10.1016/j.bcp.2017.04.015. PMID: 28431939.
Article19. Datta S, Choudhury D, Das A, Das Mukherjee D, Das N, Roy SS, Chakrabarti G. 2017; Paclitaxel resistance development is associated with biphasic changes in reactive oxygen species, mitochondrial membrane potential and autophagy with elevated energy production capacity in lung cancer cells: a chronological study. Tumour Biol. 39:1010428317694314. DOI: 10.1177/1010428317694314. PMID: 28240052.
Article20. Eum KH, Lee M. 2011; Crosstalk between autophagy and apoptosis in the regulation of paclitaxel-induced cell death in v-Ha-ras-transformed fibroblasts. Mol Cell Biochem. 348:61–68. DOI: 10.1007/s11010-010-0638-8. PMID: 21069434.
Article21. Wiedenheft B, Sternberg SH, Doudna JA. 2012; RNA-guided genetic silencing systems in bacteria and archaea. Nature. 482:331–338. DOI: 10.1038/nature10886. PMID: 22337052.
Article22. Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. 2012; A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337:816–821. DOI: 10.1126/science.1225829. PMID: 22745249. PMCID: PMC6286148.
Article23. Hammond EM, Brunet CL, Johnson GD, Parkhill J, Milner AE, Brady G, Gregory CD, Grand RJ. 1998; Homology between a human apoptosis specific protein and the product of APG5, a gene involved in autophagy in yeast. FEBS Lett. 425:391–395. DOI: 10.1016/S0014-5793(98)00266-X. PMID: 9563500.
Article24. Mizushima N, Sugita H, Yoshimori T, Ohsumi Y. 1998; A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J Biol Chem. 273:33889–33892. DOI: 10.1074/jbc.273.51.33889. PMID: 9852036.25. Ahn JH, Kim YK, Lee M. 2011; Decreased interaction of Raf-1 with its negative regulator Spry2 as a mechanism for acquired drug resistance. Biomol Ther. 19:174–180. DOI: 10.4062/biomolther.2011.19.2.174.
Article26. Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 25:402–408. DOI: 10.1006/meth.2001.1262. PMID: 11846609.27. Hwang SH, Han BI, Lee M. 2018; Knockout of ATG5 leads to malignant cell transformation and resistance to Src family kinase inhibitor PP2. J Cell Physiol. 233:506–515. DOI: 10.1002/jcp.25912. PMID: 28294316.
Article28. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. 2000; LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19:5720–5728. DOI: 10.1093/emboj/19.21.5720. PMID: 11060023. PMCID: PMC305793.
Article29. Kimura S, Noda T, Yoshimori T. 2007; Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 3:452–460. DOI: 10.4161/auto.4451. PMID: 17534139.
Article30. Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang CC, Sheffler DJ, Teriete P, Asara JM, Turk BE, Cosford ND, Shaw RJ. 2015; Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol Cell. 59:285–297. DOI: 10.1016/j.molcel.2015.05.031. PMID: 26118643. PMCID: PMC4530630.
Article31. Piao S, Amaravadi RK. 2016; Targeting the lysosome in cancer. Ann N Y Acad Sci. 1371:45–54. DOI: 10.1111/nyas.12953. PMID: 26599426. PMCID: PMC4879098.
Article32. Sun YL, Patel A, Kumar P, Chen ZS. 2012; Role of ABC transporters in cancer chemotherapy. Chin J Cancer. 31:51–57. DOI: 10.5732/cjc.011.10466. PMID: 22257384. PMCID: PMC3777472.
Article33. Kumar P, Zhang DM, Degenhardt K, Chen ZS. 2012; Autophagy and transporter-based multi-drug resistance. Cells. 1:558–575. DOI: 10.3390/cells1030558. PMID: 24710490. PMCID: PMC3901113.
Article34. Kathawala RJ, Wang YJ, Ashby CR Jr, Chen ZS. 2014; Recent advances regarding the role of ABC subfamily C member 10 (ABCC10) in the efflux of antitumor drugs. Chin J Cancer. 33:223–230. DOI: 10.5732/cjc.013.10122. PMID: 24103790. PMCID: PMC4026542.
Article35. Scripture CD, Figg WD, Sparreboom A. 2005; Paclitaxel chemotherapy: from empiricism to a mechanism-based formulation strategy. Ther Clin Risk Manag. 1:107–114. DOI: 10.2147/tcrm.1.2.107.62910. PMID: 18360550. PMCID: PMC1661618.
Article36. Eum KH, Lee M. 2011; Targeting the autophagy pathway using ectopic expression of Beclin 1 in combination with rapamycin in drug-resistant v-Ha-ras-transformed NIH 3T3 cells. Mol Cells. 31:231–238. DOI: 10.1007/s10059-011-0034-6. PMID: 21350938. PMCID: PMC3932692.
Article37. Ding R, Jin S, Pabon K, Scotto KW. 2016; A role for ABCG2 beyond drug transport: regulation of autophagy. Autophagy. 12:737–751. DOI: 10.1080/15548627.2016.1155009. PMID: 26983466. PMCID: PMC4854550.
Article38. Chen J, Zhang L, Zhou H, Wang W, Luo Y, Yang H, Yi H. 2018; Inhibition of autophagy promotes cisplatin-induced apoptotic cell death through Atg5 and Beclin 1 in A549 human lung cancer cells. Mol Med Rep. 17:6859–6865. DOI: 10.3892/mmr.2018.8686. PMID: 29512762.
Article39. Gillet JP, Gottesman MM. 2010; Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 596:47–76. DOI: 10.1007/978-1-60761-416-6_4. PMID: 19949920.
Article40. Ajabnoor GM, Crook T, Coley HM. 2012; Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 3:e260. DOI: 10.1038/cddis.2011.139. PMID: 22278287. PMCID: PMC3270273.
Article41. Sun WL, Lan D, Gan TQ, Cai ZW. 2015; Autophagy facilitates multidrug resistance development through inhibition of apoptosis in breast cancer cells. Neoplasma. 62:199–208. DOI: 10.4149/neo_2015_025. PMID: 25591585.
Article42. Yan XD, Li M, Yuan Y, Mao N, Pan LY. 2007; Biological comparison of ovarian cancer resistant cell lines to cisplatin and Taxol by two different administrations. Oncol Rep. 17:1163–1169. DOI: 10.3892/or.17.5.1163. PMID: 17390060.
Article43. Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. 1998; A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 95:15665–15670. DOI: 10.1073/pnas.95.26.15665. PMID: 9861027. PMCID: PMC28101.
Article44. Doyle L, Ross DD. 2003; Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 22:7340–7358. DOI: 10.1038/sj.onc.1206938. PMID: 14576842.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A role of Cyclosporine A that suppresses multi-drug resistance (MDR) in the secondary chemotherapy drug resistant cell line of ovarian cancer
- Paclitaxel inhibits the hyper-activation of spleen cells by lipopolysaccharide and induces cell death
- Paclitaxel-resistant cancer cell-derived secretomes elicit ABCB1-associated docetaxel cross-resistance and escape from apoptosis through FOXO3a-driven glycolytic regulation
- Mutational and Expressional Analysis of ATG5 Gene in Non-Small Cell Lung Cancers
- Clusterin confers paclitaxel resistance in ovarian cancer