Nutr Res Pract.
2020 Jun;14(3):203-217. 10.4162/nrp.2020.14.3.203.
Centella asiatica extract prevents visual impairment by promoting the production of rhodopsin in the retina
- Affiliations
-
- 1Research Institute, Genencell Co. Ltd., Yongin 16950, Korea
- 2Department of Oriental Medicine Biotechnology, College of Life Sciences, Kyung Hee University, Yongin 17104, Korea
- 3BioMedical Research Institute, Kyung Hee University, Yongin 17104, Korea
- KMID: 2502481
- DOI: http://doi.org/10.4162/nrp.2020.14.3.203
Abstract
- BACKGROUND
/OBJECTIVE: Centella asiatica, also known as Gotu kola, is a tropical medicinal plant native to Madagascar, Southeast Asia, and South Africa. It is well known to have biological activities, including wound healing, anti-inflammatory, antidiabetic, cytotoxic, and antioxidant effects. The purpose of this study was to determine the efficacy of extracts of C. asiatica against age-related eye degeneration and to examine their physiological activities.
MATERIALS/METHODS
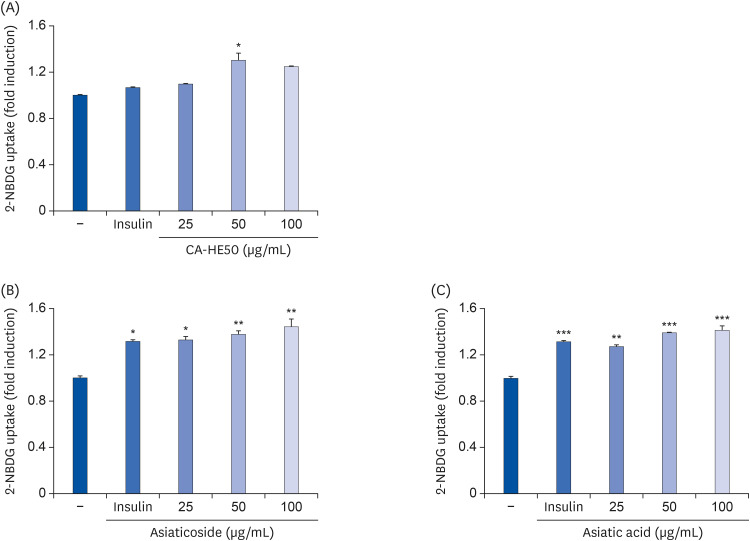
To determine the effects of CA-HE50 (C. asiatica 50% EtOH extract) on retinal pigment cells, we assessed the cytotoxicity of CoCl2 and oxidized-A2E in ARPE- 19 cells and observed the protective effects of CA-HE50 against N-methyl-N-nitrosourea (MNU)-induced retinal damage in C57BL/6 mice. In particular, we measured factors related to apoptosis and anti-oxidation and the protein levels of rhodopsin/opsin. We also measured glucose uptake to characterize glucose metabolism, a major factor in cell protection.
RESULTS
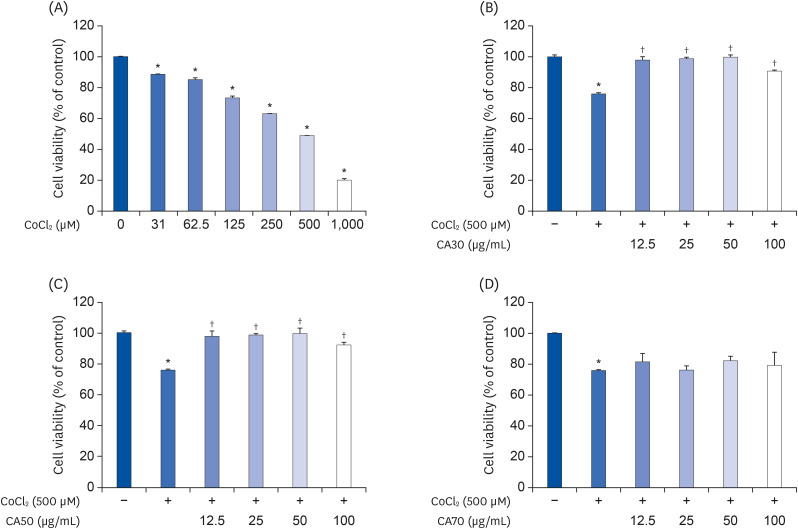
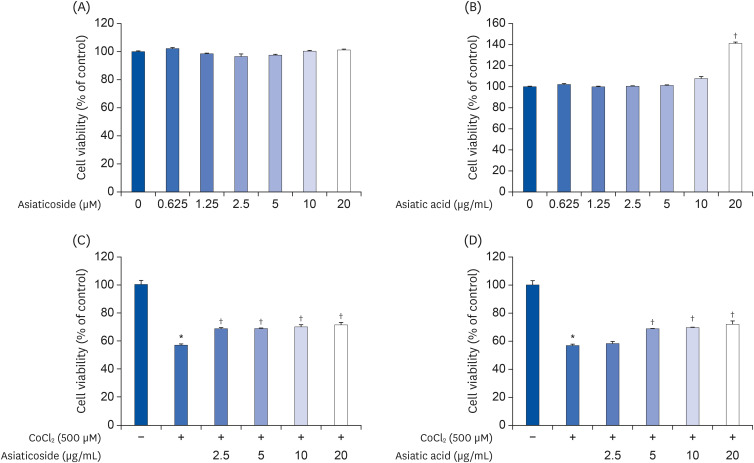
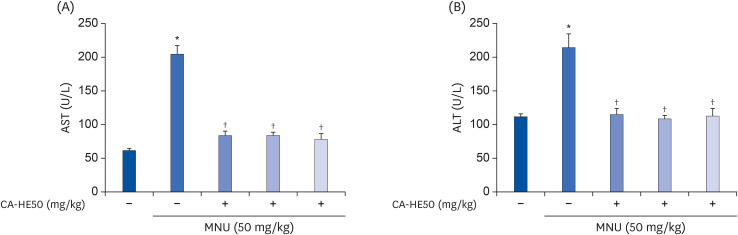
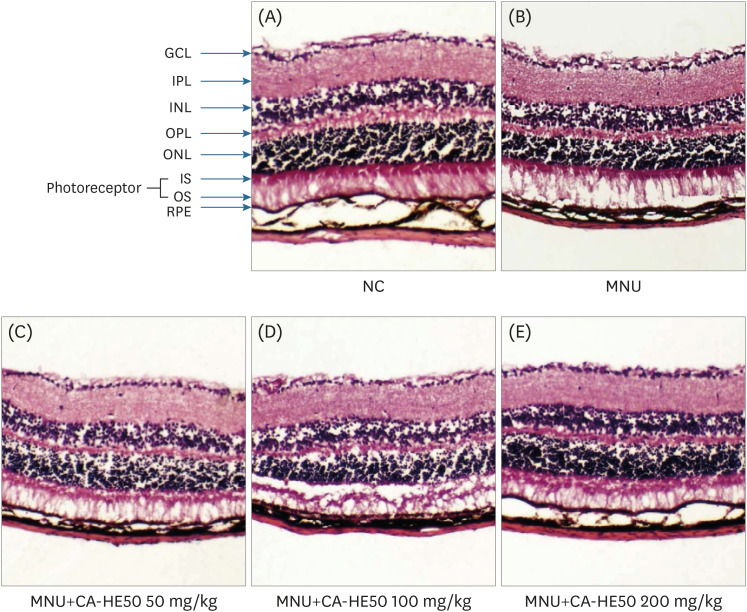
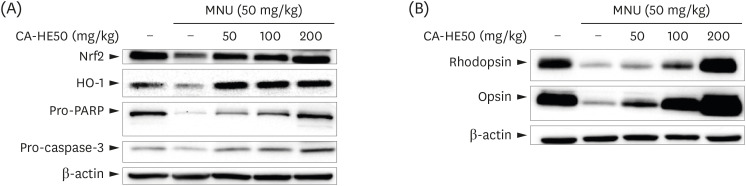
Induction of cytotoxicity with CoCl2 and oxidized-A2E inhibited decreases in the viability of ARPE-19 cells when CA-HE50 was administered, and promoted glucose uptake under normal conditions (P < 0.05). In addition, CA-HE50 inhibited degeneration/apoptosis of the retina in the context of MNU-induced toxicity (P < 0.05). In particular, CA-HE50 at 200 mg/kg inhibited the cleavage of pro-caspase-3 and pro-poly (ADP-ribose)-polymerase and maintained the expressions of nuclear factor erythroid 2-related factor 2 and heme oxygenase-1 similar to normal control levels. Rhodopsin/opsin expression was maintained at a higher level than in normal controls.
CONCLUSION
A series of experiments confirmed that CA-HE50 was effective for inhibiting or preventing age-related eye damage/degeneration. Based on these results, we believe it is worthwhile to develop drugs or functional foods related to age-related eye degeneration using CA-HE50.
Figure
Reference
-
1. Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012; 22:R741–52. PMID: 22975005.
Article2. Kumar V, Khan AA, Tripathi A, Dixit PK, Bajaj UK. Role of oxidative stress in various diseases: relevance of dietary antioxidants. J Phytopharm. 2015; 4:126–132.3. Masuda T, Shimazawa M, Hara H. Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (edaravone). Oxid Med Cell Longev. 2017; 2017:9208489. PMID: 28194256.
Article4. Benedetto MM, Contin MA. Oxidative stress in retinal degeneration promoted by constant LED light. Front Cell Neurosci. 2019; 13:139. PMID: 31105526.
Article5. Pan J, Kai G, Yuan C, Zhou B, Jin R, Yuan Y. Separation and determination of madecassic acid in triterpenic genins of Centella asiatica by high performance liquid chromatography using beta-cyclodextrin as mobile phase additive. Se Pu. 2007; 25:316–318. PMID: 17679419.6. Orhan IE. Centella asiatica (L.) urban: from traditional medicine to modern medicine with neuroprotective potential. Evid Based Complement Alternat Med. 2012; 2012:946259. PMID: 22666298.7. Somboonwong J, Kankaisre M, Tantisira B, Tantisira MH. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: an experimental animal study. BMC Complement Altern Med. 2012; 12:103. PMID: 22817824.
Article8. Bevege L. Centella asiatica: a review. Aust J Med Herbal. 2004; 16:15–27.9. Gray NE, Zweig JA, Caruso M, Martin MD, Zhu JY, Quinn JF, Soumyanath A. Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice. Brain Behav. 2018; 8:e01024. PMID: 29920983.
Article10. Anand T, Mahadeva N, Phani KG, Farhath K. Antioxidant and DNA damage preventive properties of Centella asiatica (L) Urb. Pharmacogn J. 2010; 2:53–58.
Article11. G V, K SP, V L, Rajendra W. The antiepileptic effect of Centella asiatica on the activities of Na/K, Mg and Ca-ATPases in rat brain during pentylenetetrazol-induced epilepsy. Indian J Pharmacol. 2010; 42:82–86. PMID: 20711371.12. Park JH, Choi JY, Son DJ, Park EK, Song MJ, Hellström M, Hong JT. Anti-inflammatory effect of titrated extract of Centella asiatica in phthalic anhydride-induced allergic dermatitis animal model. Int J Mol Sci. 2017; 18:E738. PMID: 28358324.
Article13. Ceremuga TE, Valdivieso D, Kenner C, Lucia A, Lathrop K, Stailey O, Bailey H, Criss J, Linton J, Fried J, Taylor A, Padron G, Johnson AD. Evaluation of the anxiolytic and antidepressant effects of asiatic acid, a compound from Gotu kola or Centella asiatica, in the male Sprague Dawley rat. AANA J. 2015; 83:91–98. PMID: 26016167.14. Maurer E, Tschopp M, Tappeiner C, Sallin P, Jazwinska A, Enzmann V. Methylnitrosourea (MNU)-induced retinal degeneration and regeneration in the zebrafish: histological and functional characteristics. J Vis Exp. 2014; 92:e51909.
Article15. Kitamoto S, Matsuyama R, Uematsu Y, Ogata K, Ota M, Yamada T, Miyata K, Funabashi H, Saito K. Optimal dose selection of N-methyl-N-nitrosourea for the rat comet assay to evaluate DNA damage in organs with different susceptibility to cytotoxicity. Mutat Res Genet Toxicol Environ Mutagen. 2015; 786-788:129–136. PMID: 26212303.
Article16. Chen YY, Liu SL, Hu DP, Xing YQ, Shen Y. N -methyl- N -nitrosourea-induced retinal degeneration in mice. Exp Eye Res. 2014; 121:102–113. PMID: 24509257.
Article17. Yamada K. Cobalt: its role in health and disease. Met Ions Life Sci. 2013; 13:295–320. PMID: 24470095.
Article18. Caltana L, Merelli A, Lazarowski A, Brusco A. Neuronal and glial alterations due to focal cortical hypoxia induced by direct cobalt chloride (CoCl2) brain injection. Neurotox Res. 2009; 15:348–358. PMID: 19384568.
Article19. Mou YH, Yang JY, Cui N, Wang JM, Hou Y, Song S, Wu CF. Effects of cobalt chloride on nitric oxide and cytokines/chemokines production in microglia. Int Immunopharmacol. 2012; 13:120–125. PMID: 22472292.
Article20. Kuehn S, Hurst J, Rensinghoff F, Tsai T, Grauthoff S, Satgunarajah Y, Dick HB, Schnichels S, Joachim SC. Degenerative effects of cobalt-chloride treatment on neurons and microglia in a porcine retina organ culture model. Exp Eye Res. 2017; 155:107–120. PMID: 28089775.
Article21. Grimm C, Willmann G. Hypoxia in the eye: a two-sided coin. High Alt Med Biol. 2012; 13:169–175. PMID: 22994516.
Article22. König J, Ott C, Hugo M, Jung T, Bulteau AL, Grune T, Höhn A. Mitochondrial contribution to lipofuscin formation. Redox Biol. 2017; 11:673–681. PMID: 28160744.
Article23. Rodolfo C, Campello S, Cecconi F. Mitophagy in neurodegenerative diseases. Neurochem Int. 2018; 117:156–166. PMID: 28797885.
Article24. Rodgers KJ, Ford JL, Brunk UT. Heat shock proteins: keys to healthy ageing? Redox Rep. 2009; 14:147–153. PMID: 19695121.
Article25. Firląg M, Kamaszewski M, Gaca K, Bałasińska B. Age-related changes in the central nervous system in selected domestic mammals and primates. Postepy Hig Med Dosw. 2013; 67:269–275.
Article26. Eldred GE, Katz ML. Fluorophores of the human retinal pigment epithelium: separation and spectral characterization. Exp Eye Res. 1988; 47:71–86. PMID: 3409988.
Article27. Sparrow JR, Zhou J, Cai B. DNA is a target of the photodynamic effects elicited in A2E-laden RPE by blue-light illumination. Invest Ophthalmol Vis Sci. 2003; 44:2245–2251. PMID: 12714667.
Article28. Holz FG, Bellman C, Staudt S, Schütt F, Völcker HE. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001; 42:1051–1056. PMID: 11274085.
Article29. Tsubura A, Yuri T, Yoshizawa K, Uehara N, Takada H. Role of fatty acids in malignancy and visual impairment: epidemiological evidence and experimental studies. Histol Histopathol. 2009; 24:223–234. PMID: 19085838.30. Nakajima M, Yuge K, Senzaki H, Shikata N, Miki H, Uyama M, Tsubura A. Photoreceptor apoptosis induced by a single systemic administration of N-methyl-N-nitrosourea in the rat retina. Am J Pathol. 1996; 148:631–641. PMID: 8579125.31. Tsubura A, Yoshizawa K, Kuwata M, Uehara N. Animal models for retinitis pigmentosa induced by MNU; disease progression, mechanisms and therapeutic trials. Histol Histopathol. 2010; 25:933–944. PMID: 20503181.32. Zulliger R, Lecaudé S, Eigeldinger-Berthou S, Wolf-Schnurrbusch UE, Enzmann V. Caspase-3-independent photoreceptor degeneration by N-methyl-N-nitrosourea (MNU) induces morphological and functional changes in the mouse retina. Graefes Arch Clin Exp Ophthalmol. 2011; 249:859–869. PMID: 21240523.
Article33. Kaarniranta K, Pawlowska E, Szczepanska J, Jablkowska A, Blasiak J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD). Int J Mol Sci. 2019; 20:2374.
Article34. Zhang C, Baffi J, Cousins SW, Csaky KG. Oxidant-induced cell death in retinal pigment epithelium cells mediated through the release of apoptosis-inducing factor. J Cell Sci. 2003; 116:1915–1923. PMID: 12668724.
Article35. Farrokh-Siar L, Rezai KA, Patel SC, van Seventer G, Ernest JT. Human fetal retinal pigment epithelium induced apoptosis of Jurkat T-cells involves caspase activation and PARP cleavage. Invest Ophthalmol Vis Sci. 2002; 43:2288.36. Bellezza I. Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Front Pharmacol. 2018; 9:1280. PMID: 30455645.
Article37. Choo YY, Lee S, Nguyen PH, Lee W, Woo MH, Min BS, Lee JH. Caffeoylglycolic acid methyl ester, a major constituent of sorghum, exhibits anti-inflammatory activity via the Nrf2/heme oxygenase-1 pathway. RSC Adv. 2015; 5:17786–17796.
Article38. Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006; 75:743–767. PMID: 16756510.
Article39. Mannu GS. Retinal phototransduction. Neurosciences. 2014; 19:275–280. PMID: 25274585.40. Zhao Y, Wieman HL, Jacobs SR, Rathmell JC. Mechanisms and methods in glucose metabolism and cell death. Methods Enzymol. 2008; 442:439–457. PMID: 18662583.41. Huang CY, Pai YC, Yu LC. Glucose-mediated cytoprotection in the gut epithelium under ischemic and hypoxic stress. Histol Histopathol. 2017; 32:543–550. PMID: 27824216.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Centella asiatica enhances neurogenesis and protects neuronal cells against H2O2-induced oxidative injury
- First Report of Septoria centellae Associated with Leaf Spot of Centella asiatica in Korea
- Antifungal Activity of Methanolic of Centella asiatica and Andrographis panicuiata
- Biological Activities and Stability of a Standardized Pentacyclic Triterpene Enriched Centella asiatica Extract
- A split-face study of moisturizer containing Centella asiatica extract after ablative fractional carbon dioxide laser resurfacing