Diabetes Metab J.
2020 Apr;44(2):286-294. 10.4093/dmj.2019.0016.
Glucose Effectiveness from Short Insulin-Modified IVGTT and Its Application to the Study of Women with Previous Gestational Diabetes Mellitus
- Affiliations
-
- 1Department of Information Engineering, Università Politecnica delle Marche, Ancona, Italy,
- 2Division of Obstetrics and Feto-Maternal Medicine, Department of Obstetrics and Gynecology, Medical University of Vienna, Vienna
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria
- 4Metabolic Unit, CNR Institute of Neuroscience, Padova, Italy
- KMID: 2502410
- DOI: http://doi.org/10.4093/dmj.2019.0016
Abstract
- Background
This study aimed to design a simple surrogate marker (i.e., predictor) of the minimal model glucose effectiveness (SG), namely calculated SG (CSG), from a short insulin-modified intravenous glucose tolerance test (IM-IVGTT), and then to apply it to study women with previous gestational diabetes mellitus (pGDM).
Methods
CSG was designed using the stepwise model selection approach on a population of subjects (n=181) ranging from normal tolerance to type 2 diabetes mellitus (T2DM). CSG was then tested on a population of women with pGDM (n=57). Each subject underwent a 3-hour IM-IVGTT; women with pGDM were observed early postpartum and after a follow-up period of up to 7 years and classified as progressors (PROG) or non-progressors (NONPROG) to T2DM. The minimal model analysis provided a reference SG.
Results
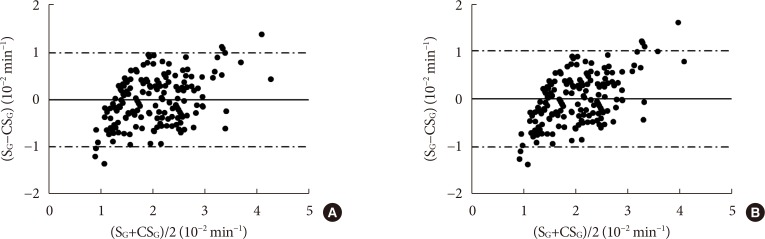
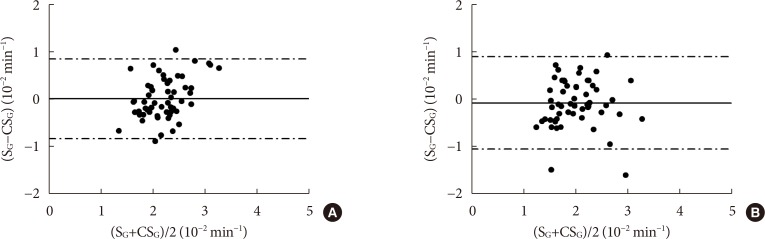
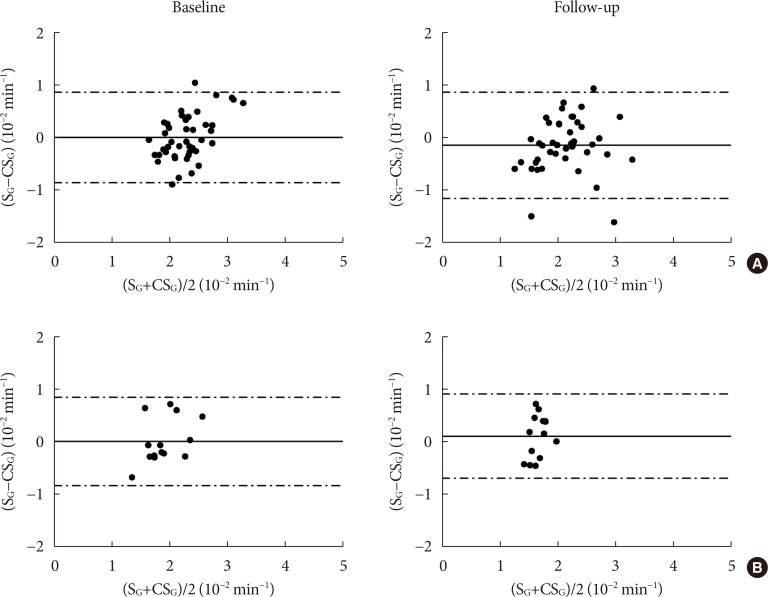
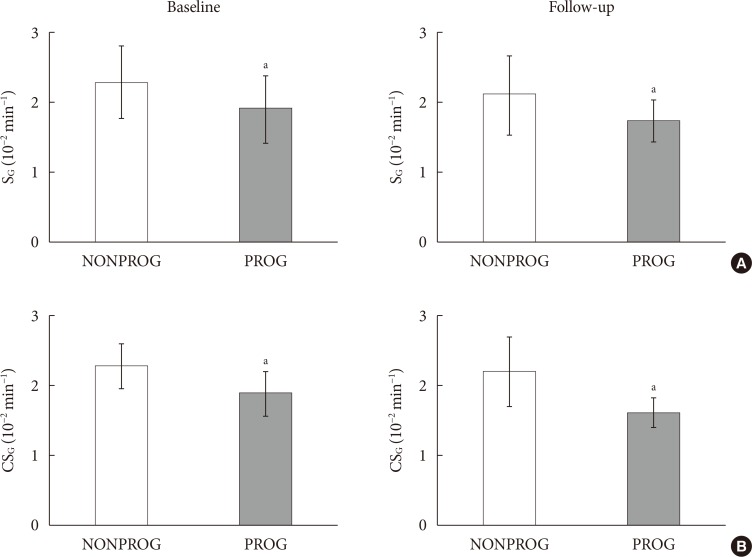
CSG was described as CSG=1.06×10–2+5.71×10–2×KG/Gpeak, KG being the mean slope (absolute value) of loge glucose in 10–25- and 25–50-minute intervals, and Gpeak being the maximum of the glucose curve. Good agreement between CSG and SG in the general population and in the pGDM group, both at baseline and follow-up (even in PROG and NONPROG subgroups), was shown by the Bland-Altman plots (<5% observations outside limits of agreement), and by the test for equivalence (equivalence margin not higher than one standard deviation). At baseline, the PROG subgroup showed significantly lower SG and CSG values compared to the NONPROG subgroup (P<0.03).
Conclusion
CSG is a valid SG predictor. In the pGDM group, glucose effectiveness appeared to be impaired in women progressing to T2DM.
Keyword
Figure
Cited by 2 articles
-
Postprandial Free Fatty Acids at Mid-Pregnancy Increase the Risk of Large-for-Gestational-Age Newborns in Women with Gestational Diabetes Mellitus
So-Yeon Kim, Young Shin Song, Soo-Kyung Kim, Yong-Wook Cho, Kyung-Soo Kim
Diabetes Metab J. 2022;46(1):140-148. doi: 10.4093/dmj.2021.0023.The Clinical Characteristics of Gestational Diabetes Mellitus in Korea: A National Health Information Database Study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Endocrinol Metab. 2021;36(3):628-636. doi: 10.3803/EnM.2020.948.
Reference
-
1. Pacini G, Thomaseth K, Ahren B. Contribution to glucose tolerance of insulin-independent vs. insulin-dependent mechanisms in mice. Am J Physiol Endocrinol Metab. 2001; 281:E693–E703. PMID: 11551845.2. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes. 1994; 43:587–592. PMID: 8138065.
Article3. Ader M, Ni TC, Bergman RN. Glucose effectiveness assessed under dynamic and steady state conditions. Comparability of uptake versus production components. J Clin Invest. 1997; 99:1187–1199. PMID: 9077526.
Article4. Alford FP, Henriksen JE, Rantzau C, Beck-Nielsen H. Glucose effectiveness is a critical pathogenic factor leading to glucose intolerance and type 2 diabetes: an ignored hypothesis. Diabetes Metab Res Rev. 2018; 34:e2989. PMID: 29451713.
Article5. Morettini M, Di Nardo F, Ingrillini L, Fioretti S, Gobl C, Kautzky-Willer A, Tura A, Pacini G, Burattini L. Glucose effectiveness and its components in relation to body mass index. Eur J Clin Invest. 2019; e13099. PMID: 30838644.
Article6. Seufert J. SGLT2 inhibitors: an insulin-independent therapeutic approach for treatment of type 2 diabetes: focus on canagliflozin. Diabetes Metab Syndr Obes. 2015; 8:543–554. PMID: 26609242.7. Winzer C, Wagner O, Festa A, Schneider B, Roden M, Bancher-Todesca D, Pacini G, Funahashi T, Kautzky-Willer A. Plasma adiponectin, insulin sensitivity, and subclinical inflammation in women with prior gestational diabetes mellitus. Diabetes Care. 2004; 27:1721–1727. PMID: 15220253.
Article8. Kautzky-Willer A, Krssak M, Winzer C, Pacini G, Tura A, Farhan S, Wagner O, Brabant G, Horn R, Stingl H, Schneider B, Waldhausl W, Roden M. Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes. 2003; 52:244–251. PMID: 12540593.
Article9. Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986; 23:113–122. PMID: 3640682.
Article10. Henriksen JE, Alford F, Ward G, Thye-Ronn P, Levin K, Hother-Nielsen O, Rantzau C, Boston R, Beck-Nielsen H. Glucose effectiveness and insulin sensitivity measurements derived from the non-insulin-assisted minimal model and the clamp techniques are concordant. Diabetes Metab Res Rev. 2010; 26:569–578. PMID: 20830736.
Article11. Morettini M, Di Nardo F, Burattini L, Fioretti S, Gobl C, Kautzky-Willer A, Pacini G, Tura A. Assessment of glucose effectiveness from short IVGTT in individuals with different degrees of glucose tolerance. Acta Diabetol. 2018; 55:1011–1018. PMID: 29931422.
Article12. Marmarelis VZ, Shin DC, Zhang Y, Kautzky-Willer A, Pacini G, D'Argenio DZ. Analysis of intravenous glucose tolerance test data using parametric and nonparametric modeling: application to a population at risk for diabetes. J Diabetes Sci Technol. 2013; 7:952–962. PMID: 23911176.
Article13. Tura A, Sbrignadello S, Succurro E, Groop L, Sesti G, Pacini G. An empirical index of insulin sensitivity from short IVGTT: validation against the minimal model and glucose clamp indices in patients with different clinical characteristics. Diabetologia. 2010; 53:144–152. PMID: 19876614.
Article14. Pacini G, Tonolo G, Sambataro M, Maioli M, Ciccarese M, Brocco E, Avogaro A, Nosadini R. Insulin sensitivity and glucose effectiveness: minimal model analysis of regular and insulin-modified FSIGT. Am J Physiol. 1998; 274:E592–E599. PMID: 9575818.15. Ludvik B, Waldhausl W, Prager R, Kautzky-Willer A, Pacini G. Mode of action of ipomoea batatas (Caiapo) in type 2 diabetic patients. Metabolism. 2003; 52:875–880. PMID: 12870164.
Article16. Avogaro A, Watanabe RM, Dall'Arche A, De Kreutzenberg SV, Tiengo A, Pacini G. Acute alcohol consumption improves insulin action without affecting insulin secretion in type 2 diabetic subjects. Diabetes Care. 2004; 27:1369–1374. PMID: 15161790.
Article17. McQuaid S, O'Gorman DJ, Yousif O, Yeow TP, Rahman Y, Gasparro D, Pacini G, Nolan JJ. Early-onset insulin-resistant diabetes in obese Caucasians has features of typical type 2 diabetes, but 3 decades earlier. Diabetes Care. 2005; 28:1216–1218. PMID: 15855595.
Article18. Basili S, Pacini G, Guagnano MT, Manigrasso MR, Santilli F, Pettinella C, Ciabattoni G, Patrono C, Davi G. Insulin resistance as a determinant of platelet activation in obese women. J Am Coll Cardiol. 2006; 48:2531–2538. PMID: 17174194.
Article19. O'Gorman DJ, Yousif O, Dixon G, McQuaid S, Murphy E, Rahman Y, Gasparro D, Pacini G, Newsholme P, Nolan JJ. In vivo and in vitro studies of GAD-antibody positive subjects with type 2 diabetes: a distinct sub-phenotype. Diabetes Res Clin Pract. 2008; 80:365–370. PMID: 18405999.20. Tura A, Grassi A, Winhofer Y, Guolo A, Pacini G, Mari A, Kautzky-Willer A. Progression to type 2 diabetes in women with former gestational diabetes: time trajectories of metabolic parameters. PLoS One. 2012; 7:e50419. PMID: 23185618.
Article21. Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van Haeften T, Renn W, Gerich J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000; 23:295–301. PMID: 10868854.
Article22. Diaconis P, Efron B. Computer-intensive methods in statistics. Sci Am. 1983; 248:116–132.
Article23. Tura A, Chemello G, Szendroedi J, Gobl C, Færch K, Vrbikova J, Pacini G, Ferrannini E, Roden M. Prediction of clamp-derived insulin sensitivity from the oral glucose insulin sensitivity index. Diabetologia. 2018; 61:1135–1141. PMID: 29484470.
Article24. Yang YJ, Youn JH, Bergman RN. Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol. 1987; 253:E595–E602. PMID: 2892414.
Article25. Finegood DT, Hramiak IM, Dupre J. A modified protocol for estimation of insulin sensitivity with the minimal model of glucose kinetics in patients with insulin-dependent diabetes. J Clin Endocrinol Metab. 1990; 70:1538–1549. PMID: 2189884.
Article26. Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990; 71:1508–1518. PMID: 2229309.
Article27. Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, Haffner SM. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010; 33:2098–2103. PMID: 20805282.
Article28. Chen YL, Lee SF, Pei C, Pei D, Lee CH, He CT, Liang YJ, Lin JD. Predicting glucose effectiveness in Chinese participants using routine measurements. Metab Syndr Relat Disord. 2016; 14:386–390. PMID: 27461066.
Article29. Thomaseth K, Brehm A, Pavan A, Pacini G, Roden M. Modeling glucose and free fatty acid kinetics during insulin-modified intravenous glucose tolerance test in healthy humans: role of counterregulatory response. Am J Physiol Regul Integr Comp Physiol. 2014; 307:R321–R331. PMID: 24848363.
Article30. Prigeon RL, Roder ME, Porte D Jr, Kahn SE. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. J Clin Invest. 1996; 97:501–507. PMID: 8567973.
Article31. Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia. 2016; 59:1403–1411. PMID: 27073002.
Article32. Svensson H, Wetterling L, Andersson-Hall U, Jennische E, Eden S, Holmang A, Lonn M. Adipose tissue and body composition in women six years after gestational diabetes: factors associated with development of type 2 diabetes. Adipocyte. 2018; 7:229–237. PMID: 30246599.
Article33. Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs: mechanisms and therapeutic implications. Nat Rev Endocrinol. 2015; 11:592–605. PMID: 26260145.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glucose Metabolism in Pregnant Women with Normal Glucose Tolerance
- Update in management of gestational diabetes mellitus
- Gestational Diabetes in Korea: Incidence and Risk Factors of Diabetes in Women with Previous Gestational Diabetes
- Nutrition Care in Gestational Diabetes Mellitus
- Insulin Secretion and Insulin Sensitivity in Women with a Previous Gestational Diabetes: Understanding of Pathogenesis of Type 2 Diabetes