J Pathol Transl Med.
2020 Mar;54(2):165-170. 10.4132/jptm.2020.02.04.
Adjunctive markers for classification and diagnosis of central nervous system tumors: results of a multi-center neuropathological survey in Korea
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Pathology, Gachon University Gil Medical Center, Incheon, Korea
- KMID: 2501606
- DOI: http://doi.org/10.4132/jptm.2020.02.04
Abstract
- Background
The revised 4th 2016 World Health Organization (WHO) classification of tumors of the central nervous system (CNS) classification has adopted integrated diagnosis encompassing the histology and molecular features of CNS tumors. We aimed to investigate the immunohistochemistry, molecular testing, and testing methods for diagnosis of CNS tumors in pathological labs of tertiary centers in Korea, and evaluate the adequacy of tests for proper diagnosis in daily practice.
Methods
A survey, composed of eight questions concerning molecular testing for diagnosis of CNS tumors, was sent to 10 neuropathologists working in tertiary centers in Korea.
Results
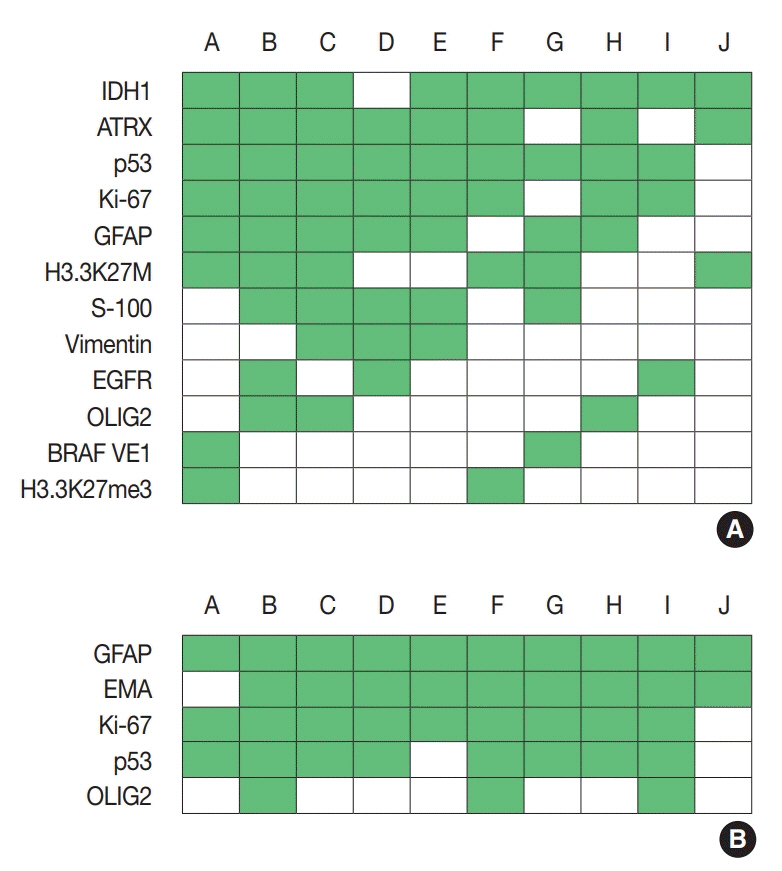
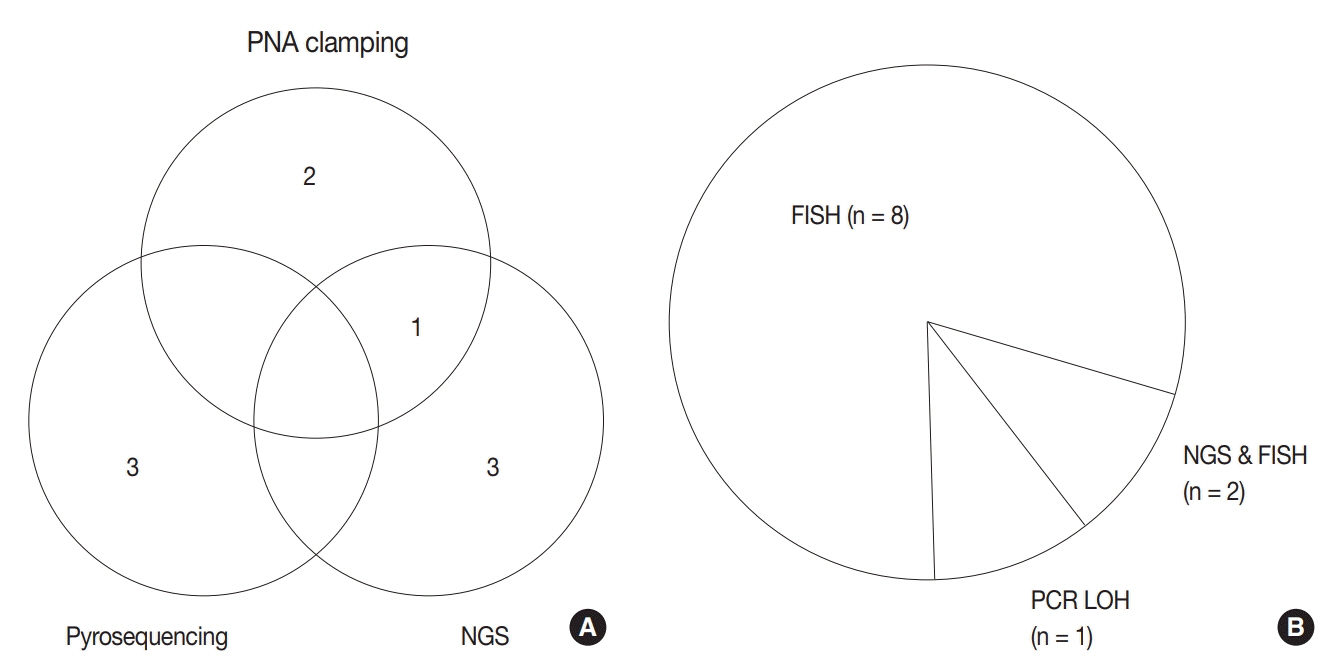
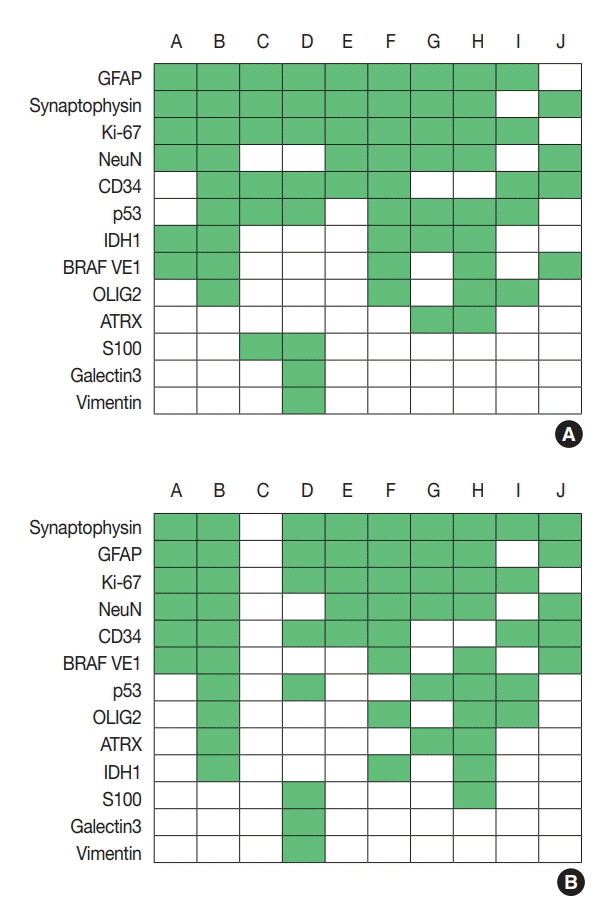
For diagnosis of astrocytic and oligodendroglial tumors, all 10 centers performed isocitrate dehydrogenase mutations testing and 1p/19q loss of heterozygosity. For glioneuronal tumors, immunohistochemistry (IHC) assays for synaptophysin (n = 9), CD34 (n = 7), BRAF(VE1) (n = 5) were used. For embryonal tumors, particularly in medulloblastoma, four respondents used IHC panel (growth factor receptor bound protein 2-associated protein 1, filamin A, and yes-associated protein 1) for molecular subclassification. Regarding meningioma, all respondents performed Ki-67 IHC and five performed telomerase reverse transcriptase promoter mutation.
Conclusions
Most tertiary centers made proper diagnosis in line with 2016 WHO classification. As classification of CNS tumors has evolved to be more complex and more ancillary tests are required, these should be performed considering the effect of necessity and justification.
Figure
Reference
-
1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. Lyon: IARC Press;2016. 4th rev.2. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360:765–73.3. Pekmezci M, Rice T, Molinaro AM, et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017; 133:1001–16.4. Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011; 333:425.5. Kannan K, Inagaki A, Silber J, et al. Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lowergrade glioma. Oncotarget. 2012; 3:1194–203.6. Leeper HE, Caron AA, Decker PA, Jenkins RB, Lachance DH, Giannini C. IDH mutation, 1p19q codeletion and ATRX loss in WHO grade II gliomas. Oncotarget. 2015; 6:30295–305.7. Liu XY, Gerges N, Korshunov A, et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012; 124:61525.8. Karsy M, Guan J, Cohen AL, Jensen RL, Colman H. New molecular considerations for glioma: IDH, ATRX, BRAF, TERT, H3 K27M. Curr Neurol Neurosci Rep. 2017; 17:19.
Article9. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015; 372:2499–508.10. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352:997–1003.11. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–66.12. Arita H, Narita Y, Fukushima S, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013; 126:267–76.13. Blumcke I, Wiestler OD. Gangliogliomas: an intriguing tumor entity associated with focal epilepsies. J Neuropathol Exp Neurol. 2002; 61:575–84.
Article14. Luyken C, Blumcke I, Fimmers R, Urbach H, Wiestler OD, Schramm J. Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer. 2004; 101:146–55.
Article15. Chappe C, Padovani L, Scavarda D, et al. Dysembryoplastic neuroepithelial tumors share with pleomorphic xanthoastrocytomas and gangliogliomas BRAF(V600E) mutation and expression. Brain Pathol. 2013; 23:574–83.16. Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011; 121:397–405.17. Lanzafame S, Torrisi A, Barbagallo G, Emmanuele C, Alberio N, Albanese V. Correlation between histological grade, MIB-1, p53, and recurrence in 69 completely resected primary intracranial meningiomas with a 6 year mean follow-up. Pathol Res Pract. 2000; 196:483–8.
Article18. Ozen O, Demirhan B, Altinors N. Correlation between histological grade and MIB-1 and p53 immunoreactivity in meningiomas. Clin Neuropathol. 2005; 24:219–24.19. Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg. 2012; 117:121–8.
Article20. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014; 24:184–9.21. Spiegl-Kreinecker S, Lotsch D, Neumayer K, et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro Oncol. 2018; 20:1584–93.22. Sahm F, Schrimpf D, Olar A, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016; 108:djv377.23. Ellison DW, Dalton J, Kocak M, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011; 121:381–96.
Article24. Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016; 131:821–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Classification and Diagnosis of Adult Glioma: A Scoping Review
- New Paradigm for the Neuropathic Pain
- Pre- and Post-Treatment Imaging of Primary Central Nervous System Tumors in the Molecular and Genetic Era
- Reclassification of Mongolian Diffuse Gliomas According to the Revised 2016 World Health Organization Central Nervous System Tumor Classification
- Adult Neurogenesis in the Central and Peripheral Nervous Systems