J Pathol Transl Med.
2020 Jan;54(1):34-44. 10.4132/jptm.2019.11.03.
HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation
- Affiliations
-
- 1Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Pathology, Kangwon National University Hospital, Chuncheon, Korea
- KMID: 2501593
- DOI: http://doi.org/10.4132/jptm.2019.11.03
Abstract
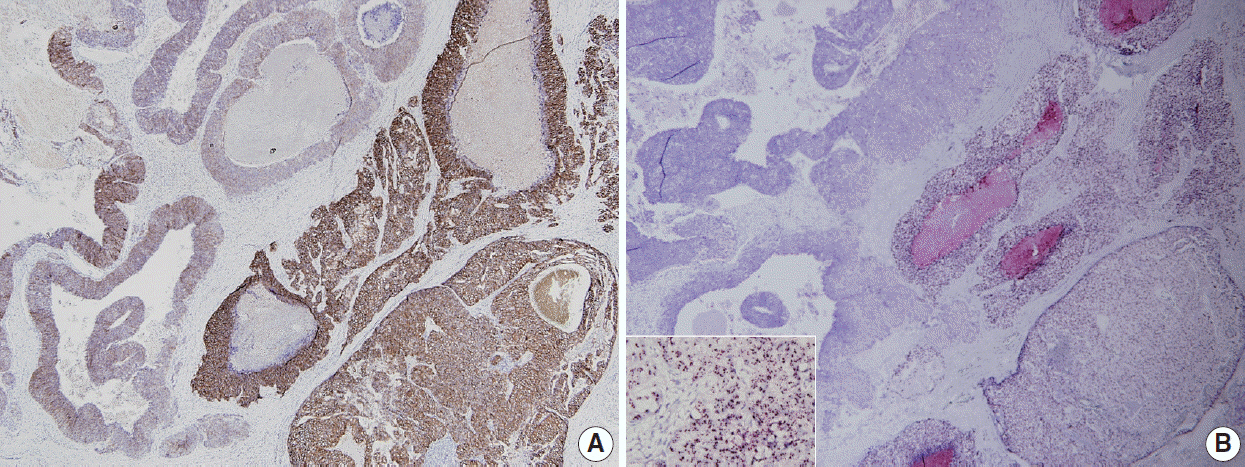
- Human epidermal growth factor receptor 2 (HER2) protein overexpression and/or HER2 gene amplification is found in about 20% of invasive breast cancers. It is a sole predictive marker for treatment benefits from HER2 targeted therapy and thus, HER2 testing is a routine practice for newly diagnosed breast cancer in pathology. Currently, HER2 immunohistochemistry (IHC) is used for a screening test, and in situ hybridization is used as a confirmation test for HER2 IHC equivocal cases. Since the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines on HER2 testing was first released in 2007, it has been updated to provide clear instructions for HER2 testing and accurate determination of HER2 status in breast cancer. During HER2 interpretation, some pitfalls such as intratumoral HER2 heterogeneity and increase in chromosome enumeration probe 17 signals may lead to inaccurate assessment of HER2 status. Moreover, HER2 status can be altered after neoadjuvant chemotherapy or during metastatic progression, due to biologic or methodologic issues. This review addresses recent updates of ASCO/CAP guidelines and factors complicating in the interpretation of HER2 status in breast cancers.
Figure
Cited by 1 articles
-
Standardized pathology report for breast cancer
Soo Youn Cho, So Yeon Park, Young Kyung Bae, Jee Yeon Kim, Eun Kyung Kim, Woo Gyeong Kim, Youngmee Kwon, Ahwon Lee, Hee Jin Lee, Ji Shin Lee, Jee Young Park, Gyungyub Gong, Hye Kyoung Yoon
J Pathol Transl Med. 2021;55(1):1-15. doi: 10.4132/jptm.2020.11.20.
Reference
-
1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235:177–82.2. Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989; 7:1120–8.
Article3. Couturier J, Vincent-Salomon A, Nicolas A, et al. Strong correlation between results of fluorescent in situ hybridization and immunohistochemistry for the assessment of the ERBB2 (HER-2/neu) gene status in breast carcinoma. Mod Pathol. 2000; 13:1238–43.
Article4. Lebeau A, Deimling D, Kaltz C, et al. Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001; 19:354–63.
Article5. Yaziji H, Goldstein LC, Barry TS, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA. 2004; 291:1972–7.
Article6. Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007; 25:118–45.7. Tanner M, Gancberg D, Di Leo A, et al. Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol. 2000; 157:1467–72.8. Dandachi N, Dietze O, Hauser-Kronberger C. Chromogenic in situ hybridization: a novel approach to a practical and sensitive method for the detection of HER2 oncogene in archival human breast carcinoma. Lab Invest. 2002; 82:1007–14.
Article9. Gong Y, Gilcrease M, Sneige N. Reliability of chromogenic in situ hybridization for detecting HER-2 gene status in breast cancer: comparison with fluorescence in situ hybridization and assessment of interobserver reproducibility. Mod Pathol. 2005; 18:1015–21.
Article10. Dietel M, Ellis IO, Hofler H, et al. Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch. 2007; 451:19–25.11. Koh YW, Lee HJ, Lee JW, Kang J, Gong G. Dual-color silver-enhanced in situ hybridization for assessing HER2 gene amplification in breast cancer. Mod Pathol. 2011; 24:794–800.12. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.13. Wolff AC, Hammond ME, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018; 36:2105–22.14. Wolff AC, Hammond ME, Hicks DG, et al. Reply to E.A. Rakha et al. J Clin Oncol. 2015; 33:1302–4.
Article15. Liu ZH, Wang K, Lin DY, et al. Impact of the updated 2018 ASCO/CAP guidelines on HER2 FISH testing in invasive breast cancer: a retrospective study of HER2 FISH results of 2233 cases. Breast Cancer Res Treat. 2019; 175:51–7.
Article16. Gordian-Arroyo AM, Zynger DL, Tozbikian GH. Impact of the 2018 ASCO/CAP HER2 Guideline Focused Update. Am J Clin Pathol. 2019; 152:17–26.
Article17. Xu B, Shen J, Guo W, Zhao W, Zhuang Y, Wang L. Impact of the 2018 ASCO/CAP HER2 guidelines update for HER2 testing by FISH in breast cancer. Pathol Res Pract. 2019; 215:251–5.
Article18. Moeder CB, Giltnane JM, Harigopal M, et al. Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol. 2007; 25:5418–25.19. Seol H, Lee HJ, Choi Y, et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol. 2012; 25:938–48.20. Lee HJ, Seo AN, Kim EJ, et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am J Clin Pathol. 2014; 142:755–66.
Article21. Lee HJ, Kim JY, Park SY, et al. Clinicopathologic significance of the intratumoral heterogeneity of HER2 gene amplification in HER2- positive breast cancer patients treated with adjuvant trastuzumab. Am J Clin Pathol. 2015; 144:570–8.22. Hou Y, Nitta H, Wei L, et al. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat. 2017; 166:447–57.
Article23. Vance GH, Barry TS, Bloom KJ, et al. Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arch Pathol Lab Med. 2009; 133:611–2.
Article24. Walker RA, Bartlett JM, Dowsett M, et al. HER2 testing in the UK: further update to recommendations. J Clin Pathol. 2008; 61:818–24.
Article25. Starczynski J, Atkey N, Connelly Y, et al. HER2 gene amplification in breast cancer: a rogues' gallery of challenging diagnostic cases: UKNEQAS interpretation guidelines and research recommendations. Am J Clin Pathol. 2012; 137:595–605.26. Hanna WM, Ruschoff J, Bilous M, et al. HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol. 2014; 27:4–18.
Article27. Lee K, Jang MH, Chung YR, et al. Prognostic significance of centromere 17 copy number gain in breast cancer depends on breast cancer subtype. Hum Pathol. 2017; 61:111–20.
Article28. Gunn S, Yeh IT, Lytvak I, et al. Clinical array-based karyotyping of breast cancer with equivocal HER2 status resolves gene copy number and reveals chromosome 17 complexity. BMC Cancer. 2010; 10:396.
Article29. Yeh IT, Martin MA, Robetorye RS, et al. Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod Pathol. 2009; 22:1169–75.
Article30. Moelans CB, de Weger RA, van Diest PJ. Absence of chromosome 17 polysomy in breast cancer: analysis by CEP17 chromogenic in situ hybridization and multiplex ligation-dependent probe amplification. Breast Cancer Res Treat. 2010; 120:1–7.
Article31. Marchio C, Lambros MB, Gugliotta P, et al. Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol. 2009; 219:16–24.32. Jang MH, Kim EJ, Kim HJ, Chung YR, Park SY. Assessment of HER2 status in invasive breast cancers with increased centromere 17 copy number. Breast Cancer Res Treat. 2015; 153:67–77.
Article33. Ma Y, Lespagnard L, Durbecq V, et al. Polysomy 17 in HER-2/neu status elaboration in breast cancer: effect on daily practice. Clin Cancer Res. 2005; 11:4393–9.
Article34. Merola R, Mottolese M, Orlandi G, et al. Analysis of aneusomy level and HER-2 gene copy number and their effect on amplification rate in breast cancer specimens read as 2+ in immunohistochemical analysis. Eur J Cancer. 2006; 42:1501–6.35. Hyun CL, Lee HE, Kim KS, et al. The effect of chromosome 17 polysomy on HER-2/neu status in breast cancer. J Clin Pathol. 2008; 61:317–21.
Article36. Downey L, Livingston RB, Koehler M, et al. Chromosome 17 polysomy without human epidermal growth factor receptor 2 amplification does not predict response to lapatinib plus paclitaxel compared with paclitaxel in metastatic breast cancer. Clin Cancer Res. 2010; 16:1281–8.
Article37. Perez EA, Reinholz MM, Hillman DW, et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol. 2010; 28:4307–15.38. Orsaria M, Khelifa S, Buza N, Kamath A, Hui P. Chromosome 17 polysomy: correlation with histological parameters and HER2NEU gene amplification. J Clin Pathol. 2013; 66:1070–5.
Article39. Krishnamurti U, Hammers JL, Atem FD, Storto PD, Silverman JF. Poor prognostic significance of unamplified chromosome 17 polysomy in invasive breast carcinoma. Mod Pathol. 2009; 22:1044–8.
Article40. Vanden Bempt I, Van Loo P, Drijkoningen M, et al. Polysomy 17 in breast cancer: clinicopathologic significance and impact on HER-2 testing. J Clin Oncol. 2008; 26:4869–74.
Article41. Bartlett JM, Munro AF, Dunn JA, et al. Predictive markers of anthracycline benefit: a prospectively planned analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601). Lancet Oncol. 2010; 11:266–74.
Article42. Tibau A, Lopez-Vilaro L, Perez-Olabarria M, et al. Chromosome 17 centromere duplication and responsiveness to anthracycline-based neoadjuvant chemotherapy in breast cancer. Neoplasia. 2014; 16:861–7.
Article43. Kim A, Shin HC, Bae YK, et al. Multiplication of chromosome 17 centromere is associated with prognosis in patients with invasive breast cancers exhibiting normal HER2 and TOP2A status. J Breast Cancer. 2012; 15:24–33.44. Nielsen KV, Ejlertsen B, Moller S, et al. Lack of independent prognostic and predictive value of centromere 17 copy number changes in breast cancer patients with known HER2 and TOP2A status. Mol Oncol. 2012; 6:88–97.
Article45. Zaczek A, Markiewicz A, Supernat A, et al. Prognostic value of TOP2A gene amplification and chromosome 17 polysomy in early breast cancer. Pathol Oncol Res. 2012; 18:885–94.46. Fountzilas G, Dafni U, Bobos M, et al. Evaluation of the prognostic role of centromere 17 gain and HER2/topoisomerase II alpha gene status and protein expression in patients with breast cancer treated with anthracycline-containing adjuvant chemotherapy: pooled analysis of two Hellenic Cooperative Oncology Group (HeCOG) phase III trials. BMC Cancer. 2013; 13:163.
Article47. Sneige N, Hess KR, Multani AS, Gong Y, Ibrahim NK. Prognostic significance of equivocal human epidermal growth factor receptor 2 results and clinical utility of alternative chromosome 17 genes in patients with invasive breast cancer: a cohort study. Cancer. 2017; 123:1115–23.
Article48. Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol. 2012; 23 Suppl 10:x231–6.
Article49. Lee SH, Chung MA, Quddus MR, Steinhoff MM, Cady B. The effect of neoadjuvant chemotherapy on estrogen and progesterone receptor expression and hormone receptor status in breast cancer. Am J Surg. 2003; 186:348–50.
Article50. Makris A, Powles TJ, Allred DC, et al. Quantitative changes in cytological molecular markers during primary medical treatment of breast cancer: a pilot study. Breast Cancer Res Treat. 1999; 53:51–9.
Article51. Zhang N, Moran MS, Huo Q, Haffty BG, Yang Q. The hormonal receptor status in breast cancer can be altered by neoadjuvant chemotherapy: a meta-analysis. Cancer Invest. 2011; 29:594–8.
Article52. Ahn S, Kim HJ, Kim M, et al. Negative conversion of progesterone receptor status after primary systemic therapy is associated with poor clinical outcome in patients with breast cancer. Cancer Res Treat. 2018; 50:1418–32.
Article53. De La Cruz LM, Harhay MO, Zhang P, Ugras S. Impact of neoadjuvant chemotherapy on breast cancer subtype: does subtype change and, if so, how?: IHC profile and neoadjuvant chemotherapy. Ann Surg Oncol. 2018; 25:3535–40.54. Xian Z, Quinones AK, Tozbikian G, Zynger DL. Breast cancer biomarkers before and after neoadjuvant chemotherapy: does repeat testing impact therapeutic management? Hum Pathol. 2017; 62:215–21.
Article55. Reddy OL, Apple SK. Breast cancer biomarker changes after neoadjuvant chemotherapy: a single institution experience and literature review. Clin Oncol. 2017; 2:1245.56. Gahlaut R, Bennett A, Fatayer H, et al. Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression: implications for the practising oncologist. Eur J Cancer. 2016; 60:40–8.57. Lim SK, Lee MH, Park IH, et al. Impact of molecular subtype conversion of breast cancers after neoadjuvant chemotherapy on clinical outcome. Cancer Res Treat. 2016; 48:133–41.
Article58. Zhou X, Zhang J, Yun H, et al. Alterations of biomarker profiles after neoadjuvant chemotherapy in breast cancer: tumor heterogeneity should be taken into consideration. Oncotarget. 2015; 6:36894–902.
Article59. Jin X, Jiang YZ, Chen S, Yu KD, Shao ZM, Di GH. Prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients: a prospective observational study. Oncotarget. 2015; 6:9600–11.
Article60. Yang YF, Liao YY, Li LQ, Xie SR, Xie YF, Peng NF. Changes in ER, PR and HER2 receptors status after neoadjuvant chemotherapy in breast cancer. Pathol Res Pract. 2013; 209:797–802.
Article61. Cockburn A, Yan J, Rahardja D, et al. Modulatory effect of neoadjuvant chemotherapy on biomarkers expression; assessment by digital image analysis and relationship to residual cancer burden in patients with invasive breast cancer. Hum Pathol. 2014; 45:249–58.
Article62. Lee HC, Ko H, Seol H, et al. Expression of immunohistochemical markers before and after neoadjuvant chemotherapy in breast carcinoma, and their use as predictors of response. J Breast Cancer. 2013; 16:395–403.
Article63. Hirata T, Shimizu C, Yonemori K, et al. Change in the hormone receptor status following administration of neoadjuvant chemotherapy and its impact on the long-term outcome in patients with primary breast cancer. Br J Cancer. 2009; 101:1529–36.
Article64. Kasami M, Uematsu T, Honda M, et al. Comparison of estrogen receptor, progesterone receptor and Her-2 status in breast cancer pre- and post-neoadjuvant chemotherapy. Breast. 2008; 17:523–7.
Article65. Neubauer H, Gall C, Vogel U, et al. Changes in tumour biological markers during primary systemic chemotherapy (PST). Anticancer Res. 2008; 28:1797–804.66. Faneyte IF, Schrama JG, Peterse JL, Remijnse PL, Rodenhuis S, van de Vijver MJ. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer. 2003; 88:406–12.
Article67. Guarneri V, Dieci MV, Barbieri E, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013; 24:2990–4.
Article68. Valent A, Penault-Llorca F, Cayre A, Kroemer G. Change in HER2 (ERBB2) gene status after taxane-based chemotherapy for breast cancer: polyploidization can lead to diagnostic pitfalls with potential impact for clinical management. Cancer Genet. 2013; 206:37–41.69. Schrijver W, Suijkerbuijk KP, van Gils CH, van der Wall E, Moelans CB, van Diest PJ. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst. 2018; 110:568–80.
Article70. de Duenas EM, Hernandez AL, Zotano AG, et al. Prospective evaluation of the conversion rate in the receptor status between primary breast cancer and metastasis: results from the GEICAM 2009-03 ConvertHER study. Breast Cancer Res Treat. 2014; 143:507–15.
Article71. Curtit E, Nerich V, Mansi L, et al. Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. Oncologist. 2013; 18:667–74.
Article72. Nakamura R, Yamamoto N, Onai Y, Watanabe Y, Kawana H, Miyazaki M. Importance of confirming HER2 overexpression of recurrence lesion in breast cancer patients. Breast Cancer. 2013; 20:336–41.
Article73. Aurilio G, Monfardini L, Rizzo S, et al. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol. 2013; 52:1649–56.
Article74. Duchnowska R, Dziadziuszko R, Trojanowski T, et al. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012; 14:R119.
Article75. Jensen JD, Knoop A, Ewertz M, Laenkholm AV. ER, HER2, and TOP2A expression in primary tumor, synchronous axillary nodes, and asynchronous metastases in breast cancer. Breast Cancer Res Treat. 2012; 132:511–21.
Article76. Bogina G, Bortesi L, Marconi M, et al. Comparison of hormonal receptor and HER-2 status between breast primary tumours and relapsing tumours: clinical implications of progesterone receptor loss. Virchows Arch. 2011; 459:1–10.
Article77. Chang HJ, Han SW, Oh DY, et al. Discordant human epidermal growth factor receptor 2 and hormone receptor status in primary and metastatic breast cancer and response to trastuzumab. Jpn J Clin Oncol. 2011; 41:593–9.
Article78. Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010; 12:R75.
Article79. Woo JW, Chung YR, Ahn S, et al. Changes in biomarker status in metastatic breast cancer and their prognostic value. J Breast Cancer. 2019; 22:439–52.
Article80. Fabi A, Di Benedetto A, Metro G, et al. HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin Cancer Res. 2011; 17:2055–64.81. Van Poznak C, Harris LN, Somerfield MR. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology clinical practice guideline. J Oncol Pract. 2015; 11:514–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Impact of the Updated Guidelines on Human Epidermal Growth Factor Receptor 2 (HER2) Testing in Breast Cancer
- HER2-Low Breast Cancer: Now and in the Future
- Comparison of Silver-Enhanced in situ Hybridization and Fluorescence in situ Hybridization for HER2 Gene Status in Breast Carcinomas
- Silver-Enhanced In Situ Hybridization as an Alternative to Fluorescence In Situ Hybridization for Assaying HER2 Amplification in Clinical Breast Cancer