Intest Res.
2020 Jan;18(1):56-68. 10.5217/ir.2019.00064.
Predictive factors for achievement of mucosal healing by budesonide 2-mg foam in ulcerative colitis: a pooled analysis of data from two clinical trials
- Affiliations
-
- 1Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
- 2Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan
- 3Medical Statistics Division, AC Medical Inc., Tokyo, Japan
- 4Clinical Development Department, EA Pharma Co., Ltd., Tokyo, Japan
- 5Medical Department, EA Pharma Co., Ltd., Tokyo, Japan
- 6Medical Research, Kissei Pharmaceutical Co., Ltd., Tokyo, Japan
- 7Department of Gastroenterology and Hepatology, and TMDU Advanced Research Institute, Tokyo Medical and Dental University, Tokyo, Japan
- KMID: 2501367
- DOI: http://doi.org/10.5217/ir.2019.00064
Abstract
- Background/Aims
Mucosal healing (MH) of distal lesions in ulcerative colitis (UC) has recently been confirmed with budesonide 2-mg foam (BF) treatment in 2 clinical trials; however, few studies have investigated the predictive factors for complete MH.
Methods
We conducted a post hoc analysis using pooled data from phase II and III clinical trials evaluating the efficacy and safety of BF for UC. Additionally, we analyzed the relationships between complete MH and baseline factors and clinical symptoms from baseline to week 6.
Results
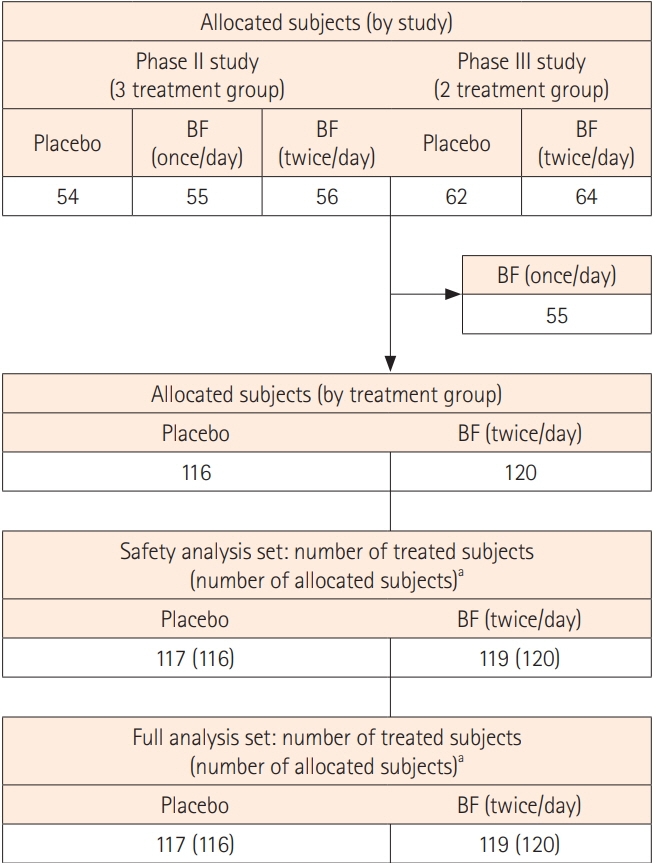
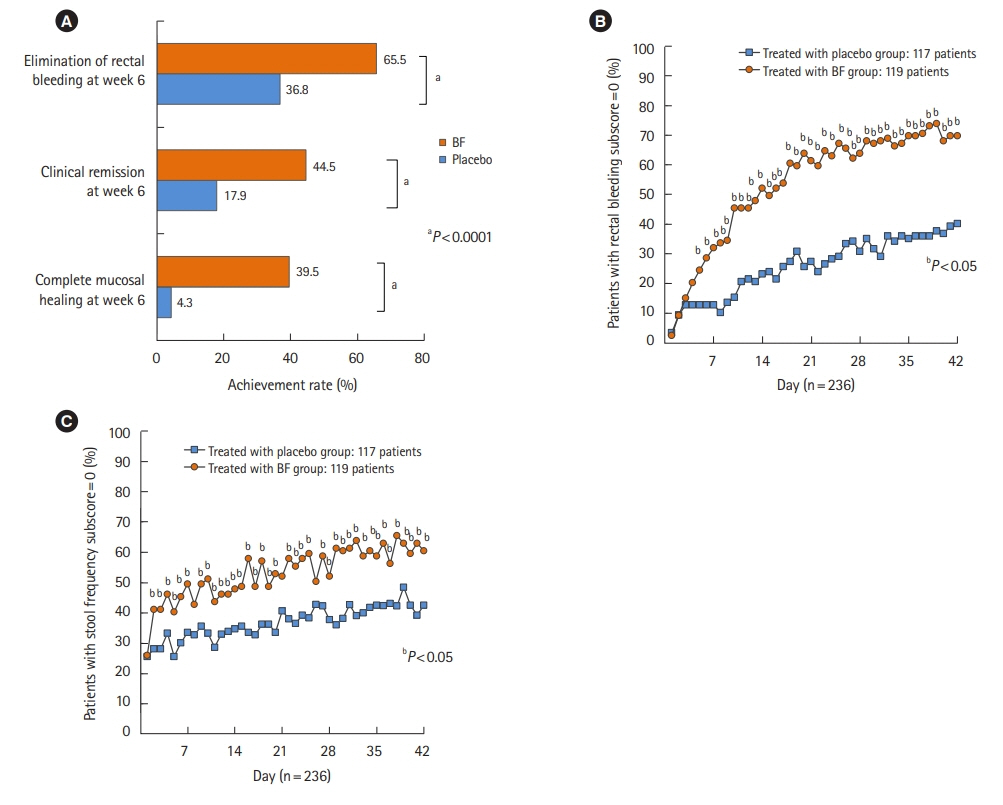
Among the 291 Japanese patients from the 2 pooled clinical studies, 119 patients in the BF twice a day group and 117 in the placebo group were included in the full analysis set. The proportion of patients with a rectal bleeding (RB) subscore of 0 was significantly higher in the BF group than in the placebo group after a 5-day treatment (P<0.05). After a 2-day treatment, significantly more patients in the BF group had a stool frequency (SF) subscore of 0 than patients in the placebo group (P<0.05). Multivariate analysis showed that complete MH at week 6 was influenced by baseline SF subscore and 5-aminosalicylic acid (5-ASA) enema or suppository use (P=0.0086 and P=0.0015, respectively). The relationship between complete MH at week 6 and RB subscore after week 2 was also confirmed.
Conclusions
Normal SF at baseline, history of 5-ASA topical product use, and elimination of RB after week 2 are suggested predictors of complete MH at week 6 with twice-daily BF treatment.
Figure
Reference
-
1. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 2: current management. J Crohns Colitis. 2017; 11:769–784.2. Ko CW, Singh S, Feuerstein JD, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. 2019; 156:748–764.3. Brunner M, Vogelsang H, Greinwald R, et al. Colonic spread and serum pharmacokinetics of budesonide foam in patients with mildly to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2005; 22:463–470.4. Hochhaus G, Derendorf H, Möllmann HW, Barth J, Hochhaus R. Pharmacodynamic aspects of glucocorticoid action. In : Mollmann HW, May B, editors. Glucocorticoid therapy in chronic inflammatory bowel disease: from basic principles to rational therapy. Dordrecht: Kluwer;1993. p. 61–79.5. Brattsand R, Thalén A, Roempke K, Källström L, Gruvstad E. Influence of 16 alpha, 17 alpha-acetal substitution and steroid nucleus fluorination on the topical to systemic activity ratio of glucocorticoids. J Steroid Biochem. 1982; 16:779–786.6. Gross V, Bar-Meir S, Lavy A, et al. Budesonide foam versus budesonide enema in active ulcerative proctitis and proctosigmoiditis. Aliment Pharmacol Ther. 2006; 23:303–312.
Article7. Suzuki Y, Iida M, Ito H, et al. 2.4 g mesalamine (Asacol 400 mg tablet) once daily is as effective as three times daily in maintenance of remission in ulcerative colitis: a randomized, noninferiority, multi-center trial. Inflamm Bowel Dis. 2017; 23:822–832.
Article8. Yoshino T, Yamakawa K, Nishimura S, Watanabe K, Yazumi S. The predictive variable regarding relapse in patients with ulcerative colitis after achieving endoscopic mucosal healing. Intest Res. 2016; 14:37–42.
Article9. Ardizzone S, Cassinotti A, Duca P, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011; 9:483–489.10. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133:412–422.
Article11. Yokoyama K, Kobayashi K, Mukae M, Sada M, Koizumi W. Clinical study of the relation between mucosal healing and long-term outcomes in ulcerative colitis. Gastroenterol Res Pract. 2013; 2013:192794.12. Naganuma M, Aoyama N, Suzuki Y, et al. Twice-daily budesonide 2-mg foam induces complete mucosal healing in patients with distal ulcerative colitis. J Crohns Colitis. 2016; 10:828–836.
Article13. Naganuma M, Aoyama N, Tada T, et al. Complete mucosal healing of distal lesions induced by twice-daily budesonide 2-mg foam promoted clinical remission of mild-to-moderate ulcerative colitis with distal active inflammation: double-blind, randomized study. J Gastroenterol. 2018; 53:494–506.14. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article15. Sandborn WJ, Kamm MA, Lichtenstein GR, Lyne A, Butler T, Joseph RE. MMX Multi Matrix System mesalazine for the induction of remission in patients with mild-to-moderate ulcerative colitis: a combined analysis of two randomized, doubleblind, placebo-controlled trials. Aliment Pharmacol Ther. 2007; 26:205–215.16. Kobayashi K, Hirai F, Naganuma M, et al. A randomized clinical trial of mesalazine suppository: the usefulness and problems of central review of evaluations of colonic mucosal findings. J Crohns Colitis. 2014; 8:1444–1453.
Article17. Höie O, Wolters F, Riis L, et al. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007; 102:1692–1701.
Article18. Package insert of budesonide 2-mg foam. https://pins.japic.or.jp/pdf/newPINS/00067157.pdf. Accessed 30 August, 2019.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Distal Ulcerative Colitis Improved by Budesonide Enemas
- The predictive variable regarding relapse in patients with ulcerative colitis after achieving endoscopic mucosal healing
- The evolving understanding of histology as an endpoint in ulcerative colitis
- Endoscopy for assessment of mucosal healing in ulcerative colitis: time bound or response guided?
- Can histologic remission be a better prognostic factor and therapeutic target beyond endoscopic mucosal healing in patients with ulcerative colitis?