Ann Pediatr Endocrinol Metab.
2020 Mar;25(1):57-62. 10.6065/apem.2020.25.1.57.
Persistent goiter with congenital hypothyroidism due to mutation in DUOXA2 gene
- Affiliations
-
- 1Department of Pediatrics, Soonchunhyang University Seoul Hospital, Seoul, Korea
- KMID: 2501039
- DOI: http://doi.org/10.6065/apem.2020.25.1.57
Abstract
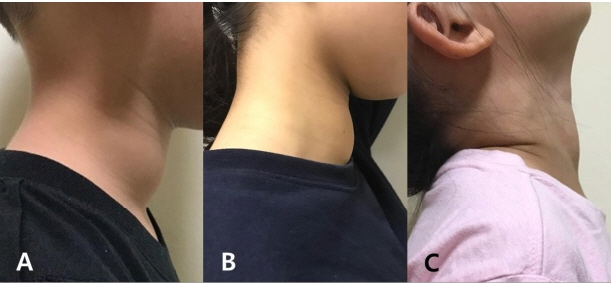
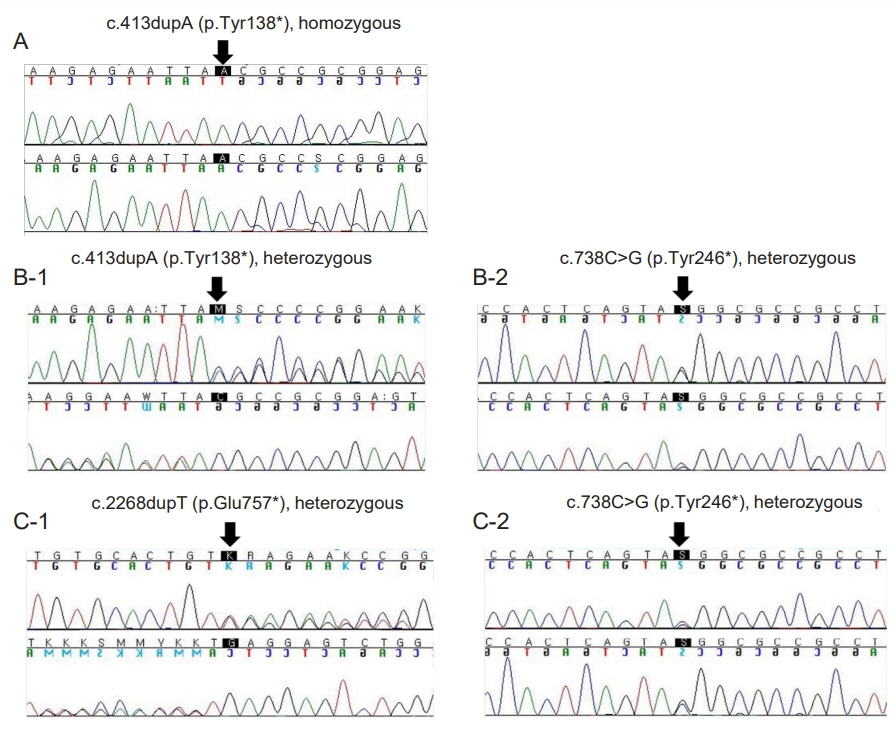
- Thyroid hormones are crucial for development of the central nervous system. Congenital hypothyroidism (CH) is the most common preventable disease resulting in mental retardation. A neonatal screening test (NST) can detect a mild form of CH that can be treated at an early age. Generally after 3 years of age, when most of the brain has matured, clinicians consider reevaluation of thyroid function for CH patients that have been identified with a normal thyroid gland at a normal position. This report presents three CH patients that developed normally, with persistent goiter despite thyroid hormone supplements. The patients’ initial thyroid-stimulating hormone (TSH) level after NST was 47, 157, and 57 mIU/L, respectively. Levothyroxine administration began at 1 or 2 months of age and was terminated after reevaluation at the age of 3, 15, and 5 years, respectively. However, 1 or 2 years later, they all resumed their medication due to increased TSH level coupled with newly developed or enlarged goiter. They all showed dual oxidase maturation factor 2 (DUOXA2) gene mutation: a homozygous mutation with DUOXA2 (c.413dupA; p.Tyr138*) in case 1, a presumed compound heterozygotic mutation with DUOXA2 (p.Tyr138*/p.Tyr246*) in case 2, and heterozygous mutations with DUOXA2 (c.738C>G; p.Tyr246*) and TPO (c.2268dupT; p.Glu757*) in case 3. When goiter persists or is newly developed despite a maintained euthyroid status, for those with transient CH history, follow-up to assess the thyroid function is recommended for at least 1 or 2 years, and genetic testing would be helpful. This study presents the first clinical cases of DUOXA2 mutation in Korea.
Keyword
Figure
Cited by 1 articles
-
Thyroid Dyshormonogenesis Due to
Dual Oxidase Maturation Factor 2 Mutation as Non-Transient Status of Hypothyroidism
Jisu Lee, Sang-gyeom Kim, Arum Oh, Heon-Seok Han
Int J Thyroidol. 2022;15(1):54-59. doi: 10.11106/ijt.2022.15.1.54.
Reference
-
References
1. LaFranchi SH. Increasing incidence of congenital hypothyroidism: some answers, more questions. J Clin Endocrinol Metab. 2011; 96:2395–7.
Article2. Maruo Y, Nagasaki K, Matsui K, Mimura Y, Mori A, Fukami M, et al. Natural course of congenital hypothyroidism by dual oxidase 2 mutations from the neonatal period through puberty. Eur J Endocrinol. 2016; 174:453–63.
Article3. Hanley P, Lord K, Bauer AJ. Thyroid disorders in children and adolescents: a review. JAMA Pediatr. 2016; 170:1008–19.4. Szinnai G. Clinical genetics of congenital hypothyroidism. Endocr Dev. 2014; 26:60–78.
Article5. Oron T, Lazar L, Ben-Yishai S, Tenenbaum A, Yackobovitch-Gavan M, Meyerovitch J, et al. Permanent vs transient congenital hypothyroidism: assessment of predictive variables. J Clin Endocrinol Metab. 2018; 103:4428–36.
Article6. Park IS, Yoon JS, So CH, Lee HS, Hwang JS. Predictors of transient congenital hypothyroidism in children with eutopic thyroid gland. Ann Pediatr Endocrinol Metab. 2017; 22:115–8.
Article7. Persani L, Rurale G, de Filippis T, Galazzi E, Muzza M, Fugazzola L. Genetics and management of congenital hypothyroidism. Best Pract Res Clin Endocrinol Metab. 2018; 32:387–96.
Article8. Park KJ, Park HK, Kim YJ, Lee KR, Park JH, Park JH, et al. DUOX2 mutations are frequently associated with congenital hypothyroidism in the Korean population. Ann Lab Med. 2016; 36:145–53.
Article9. Hulur I, Hermanns P, Nestoris C, Heger S, Refetoff S, Pohlenz J, et al. A single copy of the recently identified dual oxidase maturation factor (DUOXA) 1 gene produces only mild transient hypothyroidism in a patient with a novel biallelic DUOXA2 mutation and monoallelic DUOXA1 deletion. J Clin Endocrinol Metab. 2011; 96:E841–5.
Article10. Weber G, Rabbiosi S, Zamproni I, Fugazzola L. Genetic defects of hydrogen peroxide generation in the thyroid gland. J Endocrinol Invest. 2013; 36:261–6.11. Zamproni I, Grasberger H, Cortinovis F, Vigone MC, Chiumello G, Mora S, et al. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008; 93:605–10.
Article12. Corvilain B, van Sande J, Laurent E, Dumont JE. The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology. 1991; 128:779–85.
Article13. Liu S, Liu L, Niu X, Lu D, Xia H, Yan S. A novel missense mutation (I26M) in DUOXA2 causing congenital goiter hypothyroidism impairs NADPH oxidase activity but not protein expression. J Clin Endocrinol Metab. 2015; 100:1225–9.14. Sugisawa C, Higuchi S, Takagi M, Hasegawa Y, Taniyama M, Abe K, et al. Homozygous DUOXA2 mutation (p.Tyr138(*)) in a girl with congenital hypothyroidism and her apparently unaffected brother: case report and review of the literature. Endocr J. 2017; 64:807–12.
Article15. Yasumoto M, Inoue H, Ohashi I, Shibuya H, Onishi T. Simple new technique for sonographic measurement of the thyroid in neonates and small children. J Clin Ultrasound. 2004; 32:82–5.
Article16. Yi RH, Zhu WB, Yang LY, Lan L, Chen Y, Zhou JF, et al. A novel dual oxidase maturation factor 2 gene mutation for congenital hypothyroidism. Int J Mol Med. 2013; 31:467–70.
Article17. Tanase-Nakao K, Miyata I, Terauchi A, Saito M, Wada S, Hasegawa T, et al. Fetal goitrous hypothyroidism and polyhydramnios in a patient with compound heterozygous DUOXA2 mutations. Horm Res Paediatr. 2018; 90:132–7.
Article18. Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2009; 23:1205–18.
Article19. Moreno JC, Pauws E, van Kampen AH, Jedlicková M, de Vijlder JJ, Ris-Stalpers C. Cloning of tissue-specific genes using serial analysis of gene expression and a novel computational substraction approach. Genomics. 2001; 75:70–6.
Article20. Jin HY, Heo SH, Kim YM, Kim GH, Choi JH, Lee BH, et al. High frequency of DUOX2 mutations in transient or permanent congenital hypothyroidism with eutopic thyroid glands. Horm Res Paediatr. 2014; 82:252–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thyroid Dyshormonogenesis Due to Dual Oxidase Maturation Factor 2 Mutation as Non-Transient Status of Hypothyroidism
- A case of congenital goiter with congenital hypothyroidism due to organification defect
- Compound Heterozygous Mutations in the DUOX2/DUOXA2 Genes Cause Congenital Hypothyroidism
- A Case of Prenatal Diagnosis of Congenital Fetal Goiter in Hyperthyroidism Mother
- A family with Townes-Brocks syndrome with congenital hypothyroidism and a novel mutation of the SALL1 gene