Ann Dermatol.
2020 Apr;32(2):93-100. 10.5021/ad.2020.32.2.93.
Optimal Timing of Surgical Excision in Pediatric Pilomatricoma: Association between Clinicopathological Features and Cosmetic Outcomes
- Affiliations
-
- 1Department of Dermatology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. uuhderma@daum.net
- KMID: 2471334
- DOI: http://doi.org/10.5021/ad.2020.32.2.93
Abstract
- BACKGROUND
The treatment of choice for pilomatricomas is surgical excision; however, data for the optimal timing of treatment and cosmetic outcomes are limited.
OBJECTIVE
This study aimed to investigate the optimal timing of treatment in pilomatricomas by considering clinicopathological findings and cosmetic outcomes.
METHODS
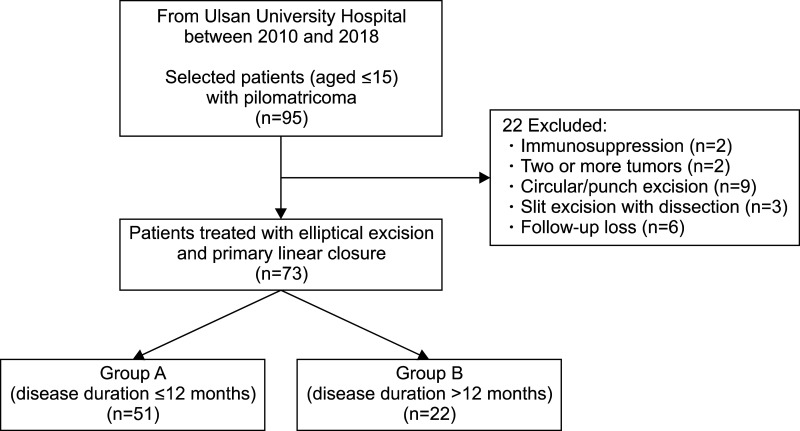
Seventy-three pilomatricomas patients aged ≤15 years were retrospectively reviewed. Patients were classified into early excision (disease duration ≤12 months, group A) and delayed excision groups (disease duration >12 months, group B). Tumor characteristics, and histopathological features with evolutionary stages were assessed. Cosmetic outcomes were evaluated by the Modified Vancouver Scar Scale (MVSS), 5-point patient satisfaction score, and complication rates.
RESULTS
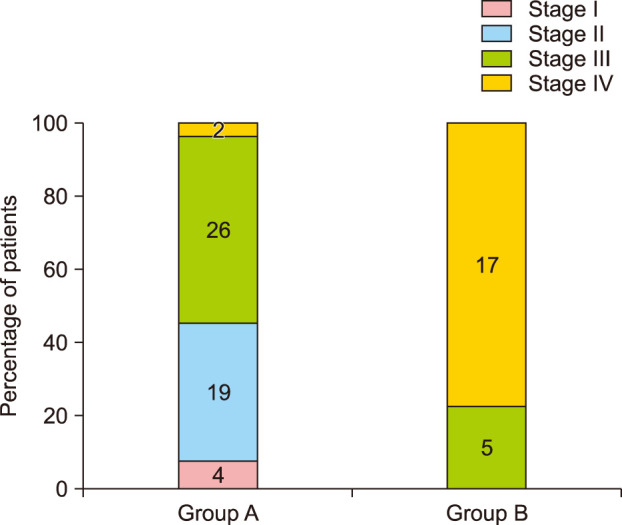
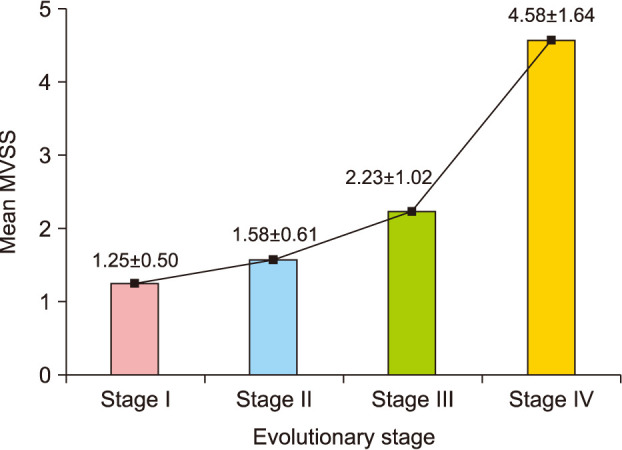
Group A showed better cosmetic outcomes than group B in the MVSS (1.53±1.22 vs. 3.68±1.84), 5-point patient satisfaction score (4.08±0.89 vs. 3.18±1.01), and complication rates (11.8% vs. 36.4%), respectively (p<0.05). Secondary anetoderma, tent sign, calcification, and late regressive stage (evolutionary stage IV) were more common in group B, (p<0.05). Moreover, evolutionary stages showed a positive correlation with mean MVSS (r=0.670, p<0.05).
CONCLUSION
Early excision (disease duration ≤12 months) provides superior cosmetic outcomes compared to delayed procedures. Early recognition, diagnosis, and management for pediatric pilomatricomas is important to improve overall cosmetic outcomes.
Figure
Reference
-
1. Julian CG, Bowers PW. A clinical review of 209 pilomatricomas. J Am Acad Dermatol. 1998; 39(2 Pt 1):191–195. PMID: 9704827.
Article2. Pirouzmanesh A, Reinisch JF, Gonzalez-Gomez I, Smith EM, Meara JG. Pilomatrixoma: a review of 346 cases. Plast Reconstr Surg. 2003; 112:1784–1789. PMID: 14663221.3. Srivastava D, Taylor RS. Appendage tumors and hamartomas of the skin. In : Fitzpatrick TB, Goldsmith LA, editors. Fitzpatrick's dermatology in general medicine. Vol. 1. 8th ed. New York: McGraw-Hill Medical;2012.4. Jones CD, Ho W, Robertson BF, Gunn E, Morley S. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631–641. PMID: 30119102.
Article5. Kaddu S, Soyer HP, Hödl S, Kerl H. Morphological stages of pilomatricoma. Am J Dermatopathol. 1996; 18:333–338. PMID: 8879294.
Article6. Pant I, Joshi SC, Kaur G, Kumar G. Pilomatricoma as a diagnostic pitfall in clinical practice: report of two cases and review of literature. Indian J Dermatol. 2010; 55:390–392. PMID: 21430899.
Article7. Yencha MW. Head and neck pilomatricoma in the pediatric age group: a retrospective study and literature review. Int J Pediatr Otorhinolaryngol. 2001; 57:123–128. PMID: 11165649.
Article8. Alli N, Güngör E, Artüz F. Perforating pilomatricoma. J Am Acad Dermatol. 1996; 35:116–118. PMID: 8682949.
Article9. Honda Y, Oh-i T, Koga M, Tokuda Y. Perforating pilomatricoma: transepithelial elimination or not. J Dermatol. 2002; 29:100–103. PMID: 11890292.
Article10. Fujioka M, Gozo N, Osamu M, Tsuneyuki Y, Takehisa Y. Secondary anetoderma overlying pilomatrixomas. Dermatology. 2003; 207:316–318. PMID: 14571077.
Article11. Ohnishi T, Nakamura Y, Watanabe S. Perforating pilomatricoma in a process of total elimination. J Am Acad Dermatol. 2003; 49(2 Suppl Case Reports):S146–S147. PMID: 12894105.
Article12. Miura T, Yamamoto T. Perforating pilomatricoma with anetodermic epidermis in an adolescent with lymphoma. Pediatr Dermatol. 2013; 30:e68–e69. PMID: 22937738.
Article13. Cho S, Whang KK, Hahm JH. Pilomatricoma: a clinical and histopathologic study of 13 cases. Ann Dermatol. 2000; 12:179–184.
Article14. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010; 10:e43. PMID: 20596233.15. Bae SH, Bae YC. Analysis of frequency of use of different scar assessment scales based on the scar condition and treatment method. Arch Plast Surg. 2014; 41:111–115. PMID: 24665417.
Article16. Hernández-Núñez A, Nájera Botello L, Romero Maté A, Martínez-Sánchez C, Utrera Busquets M, Calderón Komáromy A, et al. Retrospective study of pilomatricoma: 261 tumors in 239 patients. Actas Dermosifiliogr. 2014; 105:699–705. PMID: 24838222.
Article17. Kwon D, Grekov K, Krishnan M, Dyleski R. Characteristics of pilomatrixoma in children: a review of 137 patients. Int J Pediatr Otorhinolaryngol. 2014; 78:1337–1341. PMID: 24954158.
Article18. Han G, Kim AR, Song HJ, Oh CH, Jeon J. Updated view on epidemiology and clinical aspects of pilomatricoma in adults. Int J Dermatol. 2017; 56:1032–1036. PMID: 28895117.
Article19. Kaddu S, Soyer HP, Cerroni L, Salmhofer W, Hödl S. Clinical and histopathologic spectrum of pilomatricomas in adults. Int J Dermatol. 1994; 33:705–708. PMID: 8002139.
Article20. Kim JE, Sohn KM, Woo YJ, Jeong KH, Kim M, Lee JD, et al. A clinicoimmunohistopathologic study of anetoderma: is protruding type more advanced in stage than indented type? J Immunol Res. 2016; 2016:4325463. PMID: 28116317.
Article21. Venencie PY, Winkelmann RK, Moore BA. Anetoderma. Clinical findings, associations, and long-term follow-up evaluations. Arch Dermatol. 1984; 120:1032–1039. PMID: 6465909.
Article22. Graham JL, Merwin CF. The tent sign of pilomatricoma. Cutis. 1978; 22:577–580. PMID: 729402.23. Hwang JY, Lee SW, Lee SM. The common ultrasonographic features of pilomatricoma. J Ultrasound Med. 2005; 24:1397–1402. PMID: 16179624.
Article24. Touart DM, Sau P. Cutaneous deposition diseases. Part II. J Am Acad Dermatol. 1998; 39(4 Pt 1):527–544. quiz 545-546. PMID: 9777759.
Article25. Walsh JS, Fairley JA. Calcifying disorders of the skin. J Am Acad Dermatol. 1995; 33(5 Pt 1):693–706. quiz 707-710. PMID: 7593766.
Article26. Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E. Calcinosis cutis: part I. Diagnostic pathway. J Am Acad Dermatol. 2011; 65:1–12. quiz 13-14. PMID: 21679810.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of a Hybrid Cyst Composed of an Epidermal Cyst and a Pilomatricoma
- Pediatric Inguinal Hernia Surgery: Recent Strategies and Techniques
- Pilomatricoma of the Subauricular Region: Report of Case
- Malignant pilomatricoma of the cheek in an infant
- Pilomatricoma of the preauricular region: Report of a case