Korean J Physiol Pharmacol.
2020 Mar;24(2):185-191. 10.4196/kjpp.2020.24.2.185.
Association between interstitial cells of Cajal and anti-vinculin antibody in human stomach
- Affiliations
-
- 1Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon 24289, Korea. schlp@hanmail.net
- 2Department of Pathology, Kangwon National University School of Medicine, Chuncheon 24289, Korea.
- 3Kangwon National University Institute of Medical Science, Chuncheon 24289, Korea.
- KMID: 2471040
- DOI: http://doi.org/10.4196/kjpp.2020.24.2.185
Abstract
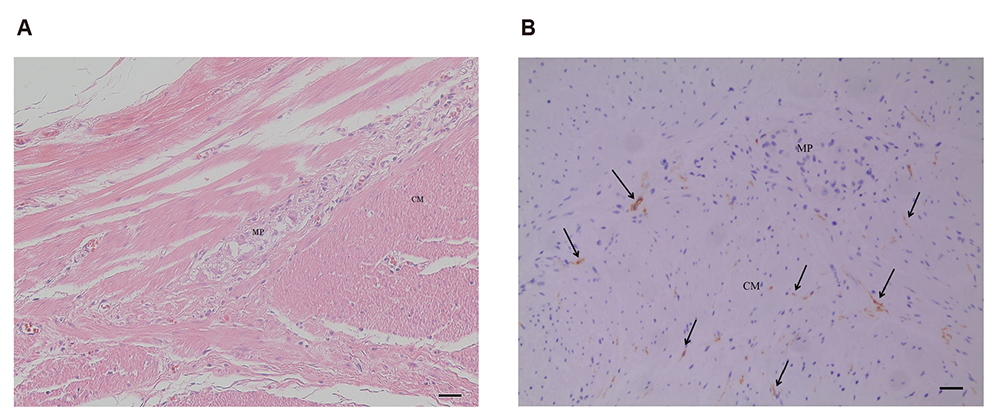
- Interstitial cells of Cajal (ICC) are known as the pacemaker cells of gastrointestinal tract, and it has been reported that acute gastroenteritis induces intestinal dysmotility through antibody to vinculin, a cytoskeletal protein in gut, resulting in small intestinal bacterial overgrowth, so that anti-vinculin antibody can be used as a biomarker for irritable bowel syndrome. This study aimed to determine correlation between serum anti-vinculin antibody and ICC density in human stomach. Gastric specimens from 45 patients with gastric cancer who received gastric surgery at Kangwon National University Hospital from 2013 to 2017 were used. ICC in inner circular muscle, and myenteric plexus were counted. Corresponding patient's blood samples were used to determine the amount of anti-vinculin antibody by enzyme-linked immunosorbent assay. Analysis was done to determine correlation between anti-vinculin antibody and ICC numbers. Patients with elevated anti-vinculin antibody titer (above median value) had significantly lower number of ICC in inner circular muscle (71.0 vs. 240.5, p = 0.047), and myenteric plexus (12.0 vs. 68.5, p < 0.01) compared to patients with lower anti-vinculin antibody titer. Level of serum anti-vinculin antibody correlated significantly with density of ICC in myenteric plexus (r = −0.379, p = 0.01; Spearman correlation). Increased level of circulating anti-vinculin antibody was significantly correlated with decreased density of ICC in myenteric plexus of human stomach.
MeSH Terms
Figure
Reference
-
1. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014; 505:559–563.
Article2. Ford AC, Thabane M, Collins SM, Moayyedi P, Garg AX, Clark WF, Marshall JK. Prevalence of uninvestigated dyspepsia 8 years after a large waterborne outbreak of bacterial dysentery: a cohort study. Gastroenterology. 2010; 138:1727–1736. quiz e12.
Article3. Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome--a meta-analysis. Am J Gastroenterol. 2006; 101:1894–1899. quiz 1942.
Article4. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012; 10:712–721.e4.
Article5. Mearin F, Pérez-Oliveras M, Perelló A, Vinyet J, Ibañez A, Coderch J, Perona M. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005; 129:98–104.
Article6. Saps M, Pensabene L, Di Martino L, Staiano A, Wechsler J, Zheng X, Di Lorenzo C. Post-infectious functional gastrointestinal disorders in children. J Pediatr. 2008; 152:812–816. 816.e1
Article7. Porter CK, Choi D, Cash B, Pimentel M, Murray J, May L, Riddle MS. Pathogen-specific risk of chronic gastrointestinal disorders following bacterial causes of foodborne illness. BMC Gastroenterol. 2013; 13:46.
Article8. Jee SR, Morales W, Low K, Chang C, Zhu A, Pokkunuri V, Chatterjee S, Soffer E, Conklin JL, Pimentel M. ICC density predicts bacterial overgrowth in a rat model of post-infectious IBS. World J Gastroenterol. 2010; 16:3680–3686.
Article9. Nieuwenhuijs VB, Verheem A, van Duijvenbode-Beumer H, Visser MR, Verhoef J, Gooszen HG, Akkermans LM. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg. 1998; 228:188–193.
Article10. Pimentel M, Chatterjee S, Chang C, Low K, Song Y, Liu C, Morales W, Ali L, Lezcano S, Conklin J, Finegold S. A new rat model links two contemporary theories in irritable bowel syndrome. Dig Dis Sci. 2008; 53:982–989.
Article11. Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002; 47:2639–2643.12. Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977; 59:1158–1166.
Article13. Pimentel M, Morales W, Pokkunuri V, Brikos C, Kim SM, Kim SE, Triantafyllou K, Weitsman S, Marsh Z, Marsh E, Chua KS, Srinivasan S, Barlow GM, Chang C. Autoimmunity links vinculin to the pathophysiology of chronic functional bowel changes following campylobacter jejuni infection in a rat Model. Dig Dis Sci. 2015; 60:1195–1205.
Article14. Bays JL, DeMali KA. Vinculin in cell-cell and cell-matrix adhesions. Cell Mol Life Sci. 2017; 74:2999–3009.
Article15. Yin J, Chen JD. Roles of interstitial cells of Cajal in regulating gastrointestinal motility: in vitro versus in vivo studies. J Cell Mol Med. 2008; 12:1118–1129.16. Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, Kendrick ML, Shen KR, Cima RR, Dozois EJ, Larson DW, Ordog T, Pozo MJ, Farrugia G. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil. 2011; 23:36–44.
Article17. Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003; 98:618–624.
Article18. Porcher C, Baldo M, Henry M, Orsoni P, Julé Y, Ward SM. Deficiency of interstitial cells of Cajal in the small intestine of patients with Crohn's disease. Am J Gastroenterol. 2002; 97:118–125.
Article19. Horváth VJ, Vittal H, Lörincz A, Chen H, Almeida-Porada G, Redelman D, Ordög T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006; 130:759–770.
Article20. Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, Hishida A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006; 41:1076–1087.
Article21. Yun HY, Sung R, Kim YC, Choi W, Kim HS, Kim H, Lee GJ, You RY, Park SM, Yun SJ, Kim MJ, Kim WS, Song YJ, Xu WX, Lee SJ. Regional distribution of interstitial cells of Cajal (ICC) in human stomach. Korean J Physiol Pharmacol. 2010; 14:317–324.
Article22. Lee CH, Liang CW, Espinosa I. The utility of discovered on gastrointestinal stromal tumor 1 (DOG1) antibody in surgical pathologythe GIST of it. Adv Anat Pathol. 2010; 17:222–232.
Article23. Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009; 296:G1370–G1381.
Article24. Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009; 587(Pt 20):4887–4904.
Article25. Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009; 587(Pt 20):4905–4918.26. Critchley DR. Cytoskeletal proteins talin and vinculin in integrinmediated adhesion. Biochem Soc Trans. 2004; 32(Pt 5):831–836.
Article27. Rezaie A, Park SC, Morales W, Marsh E, Lembo A, Kim JH, Weitsman S, Chua KS, Barlow GM, Pimentel M. Assessment of anti-vinculin and anti-cytolethal distending toxin B antibodies in subtypes of irritable bowel syndrome. Dig Dis Sci. 2017; 62:1480–1485.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is This the Era of Interstitial Cells of Cajal Transplantation?
- Interstitial Cells of Cajal and GI Motility
- Aging Decreases the Density of Colonic Interstitial Cells of Cajal Associated With Constipation in Rats
- The Important Roles of Interstitial Cells of Cajal and Cholinergic Receptors on Diabetes Related Dysfunction of Colon
- Injury of Partial Colon Obstruction in Colonic Interstitial Cells With/Without Interstitial Cells of Cajal