J Gynecol Oncol.
2020 Mar;31(2):e12. 10.3802/jgo.2020.31.e12.
Efficacy and safety of neoadjuvant chemotherapy versus primary debulking surgery in patients with ovarian cancer: a meta-analysis
- Affiliations
-
- 1Department of Obstetrics and Gynecology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450052, China. superlxf1992@outlook.com
- 2Department of Imaging, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450052, China.
- KMID: 2470865
- DOI: http://doi.org/10.3802/jgo.2020.31.e12
Abstract
OBJECTIVE
Neoadjuvant chemotherapy (NACT) for the treatment of epithelial ovarian cancer (EOC) has remained controversial. This meta-analysis was performed to systematically assess the efficacy and safety of NACT versus primary debulking surgery (PDS) in patients with EOC.
METHODS
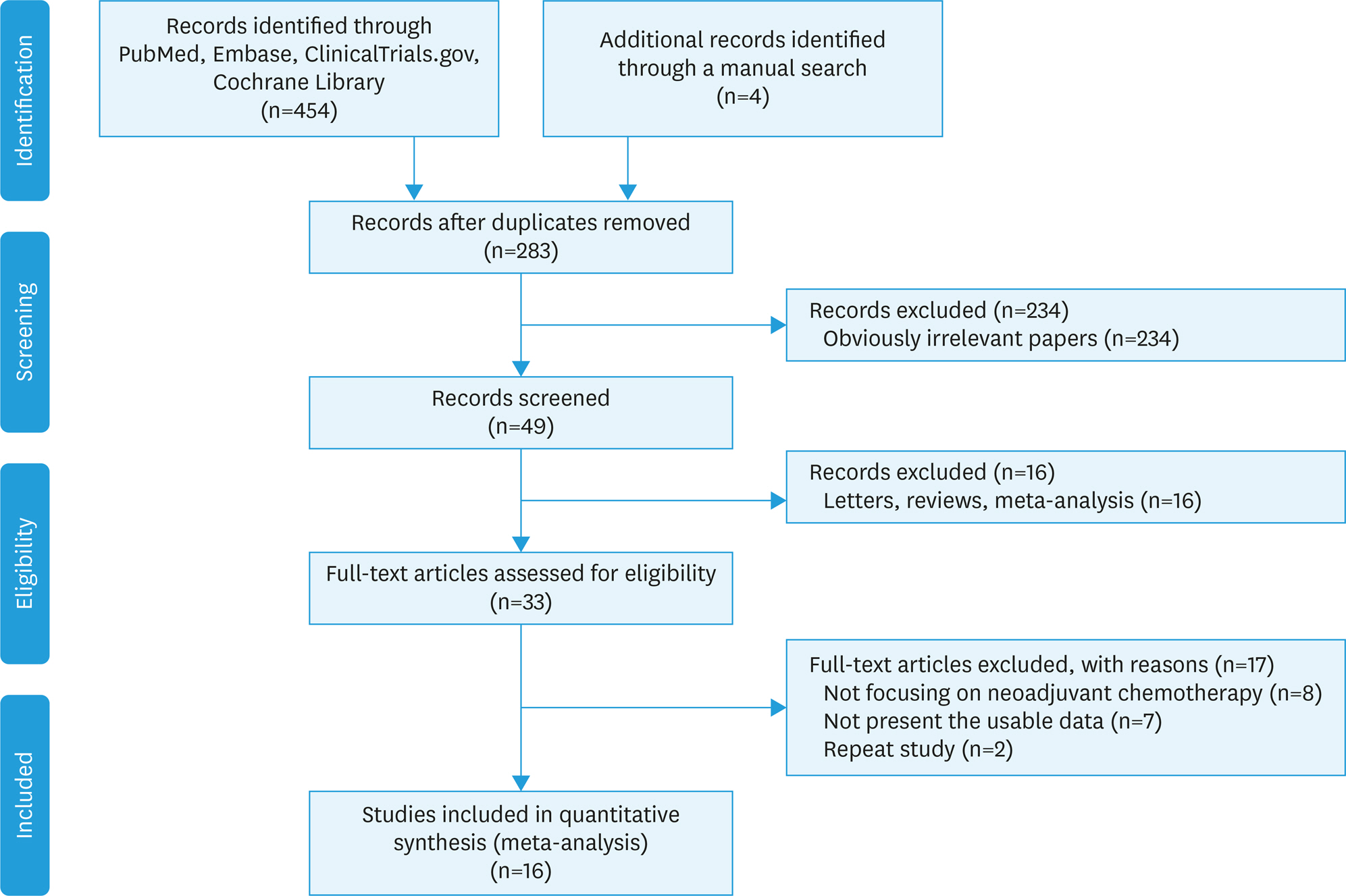
PubMed, Embase, ClinicalTrials.gov, and Cochrane Library were queried to assess the therapeutic value of NACT versus PDS in EOC. Electronic databases were queried by using the keywords "ovarian cancer/neoplasms", "primary debulking surgery", and "neoadjuvant chemotherapy".
RESULTS
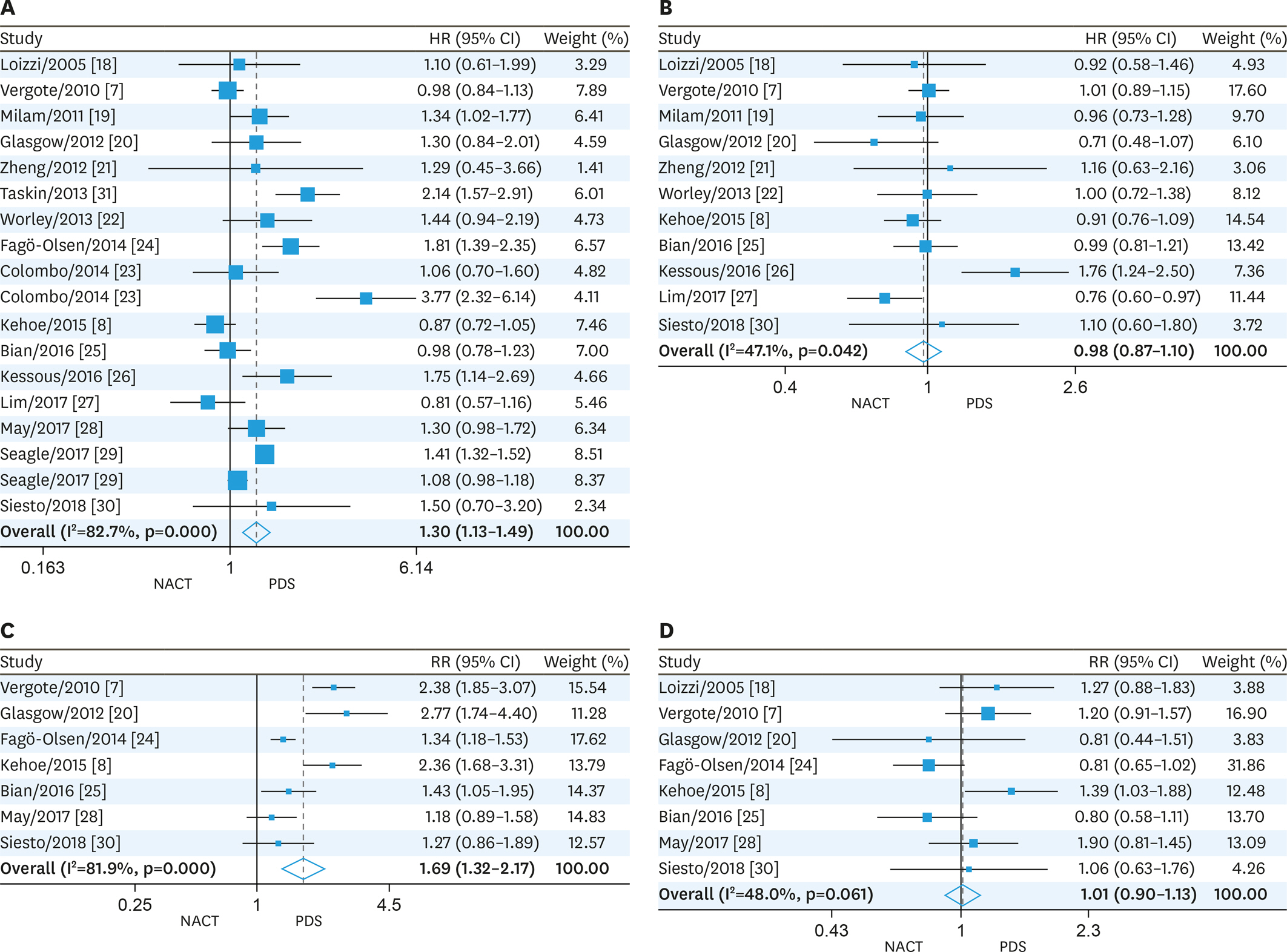
The available trials were pooled, and hazard ratios (HRs), relative risk ratios (RRs) and associated 95% confidence intervals (95% CIs) were determined. Sixteen trials involving 57,450 participants with EOC (NACT, 9,475; PDS, 47,975) were evaluated. We found that NACT resulted in markedly decreased overall survival than PDS in patients with EOC (HR=1.30; 95% CI=1.13-1.49; heterogeneity: p<0.001, ²=82.7%). Furthermore, our results demonstrated that the NACT group displayed increased completeness of debulking removal (RR=1.69, 95% CI=1.32-2.17; heterogeneity: p<0.001, ²=81.9%), and reduced risk of postsurgical death (RR=0.18, 95% CI=0.06-0.51; heterogeneity: p=0.698, ²=0%) and major infection (RR=0.29, 95% CI=0.17-0.51; heterogeneity: p=0.777, ²=0%) compared with patients administered PDS.
CONCLUSIONS
This meta-analysis indicated that NACT results in increased completeness of debulking removal, and reduced risk of postsurgical death and major infection compared with PDS, while PDS is associated with improved survival in comparison with NACT in EOC patients. TRIAL REGISTRATION: PROSPERO Identifier: CRD42019120625
MeSH Terms
Figure
Reference
-
References
1. Siegel RL, Fedewa SA, Miller KD, Goding-Sauer A, Pinheiro PS, Martinez-Tyson D, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2015; 65:457–80.
Article2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–32.
Article4. Ozols RF. Treatment goals in ovarian cancer. Int J Gynecol Cancer. 2005; 15(Suppl 1):3–11.
Article5. Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007; 25:2873–83.
Article6. Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011; 21:750–5.
Article7. Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010; 363:943–53.
Article8. Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015; 386:249–57.
Article9. McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996; 334:1–6.
Article10. van der Burg ME, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. N Engl J Med. 1995; 332:629–34.
Article11. Yang L, Zhang B, Xing G, Du J, Yang B, Yuan Q, et al. Neoadjuvant chemotherapy versus primary debulking surgery in advanced epithelial ovarian cancer: a meta-analysis of perioperative outcome. PLoS One. 2017; 12:e0186725.
Article12. Zeng LJ, Xiang CL, Gong YZ, Kuang Y, Lu FF, Yi SY, et al. Neoadjuvant chemotherapy for patients with advanced epithelial ovarian cancer: a meta-analysis. Sci Rep. 2016; 6:35914.
Article13. Moher D, Liberati A, Tetzlaff J. Altman DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–9.
Article14. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–5.
Article16. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998; 17:2815–34.
Article17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.
Article18. Loizzi V, Cormio G, Resta L, Rossi CA, Di Gilio AR, Cuccovillo A, et al. Neoadjuvant chemotherapy in advanced ovarian cancer: a case-control study. Int J Gynecol Cancer. 2005; 15:217–23.
Article19. Milam MR, Tao X, Coleman RL, Harrell R, Bassett R, Dos Reis R, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011; 21:66–71.
Article20. Glasgow MA, Yu H, Rutherford TJ, Azodi M, Silasi DA, Santin AD, et al. Neoadjuvant chemotherapy (NACT) is an effective way of managing elderly women with advanced stage ovarian cancer (FIGO Stage IIIC and IV). J Surg Oncol. 2013; 107:195–200.
Article21. Zheng H, Gao YN. Primary debulking surgery or neoadjuvant chemotherapy followed by interval debulking surgery for patients with advanced ovarian cancer. Chin J Cancer Res. 2012; 24:304–9.
Article22. Worley MJ Jr, Guseh SH, Rauh-Hain JA, Williams KA, Muto MG, Feltmate CM, et al. Does neoadjuvant chemotherapy decrease the risk of hospital readmission following debulking surgery? Gynecol Oncol. 2013; 129:69–73.
Article23. Colombo PE, Labaki M, Fabbro M, Bertrand M, Mourregot A, Gutowski M, et al. Impact of neoadjuvant chemotherapy cycles prior to interval surgery in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 2014; 135:223–30.
Article24. Fagö-Olsen CL, Ottesen B, Kehlet H, Antonsen SL, Christensen IJ, Markauskas A, et al. Does neoadjuvant chemotherapy impair long-term survival for ovarian cancer patients? A nationwide Danish study. Gynecol Oncol. 2014; 132:292–8.
Article25. Bian C, Yao K, Li L, Yi T, Zhao X. Primary debulking surgery vs. neoadjuvant chemotherapy followed by interval debulking surgery for patients with advanced ovarian cancer. Arch Gynecol Obstet. 2016; 293:163–8.
Article26. Kessous R, Laskov I, Abitbol J, Bitharas J, Yasmeen A, Salvador S, et al. Clinical outcome of neoadjuvant chemotherapy for advanced ovarian cancer. Gynecol Oncol. 2017; 144:474–9.
Article27. Lim MC, Yoo HJ, Song YJ, Seo SS, Kang S, Kim SH, et al. Survival outcomes after extensive cytoreductive surgery and selective neoadjuvant chemotherapy according to institutional criteria in bulky stage IIIC and IV epithelial ovarian cancer. J Gynecol Oncol. 2017; 28:e48.
Article28. May T, Comeau R, Sun P, Kotsopoulos J, Narod SA, Rosen B, et al. A comparison of survival outcomes in advanced serous ovarian cancer patients treated with primary debulking surgery versus neoadjuvant chemotherapy. Int J Gynecol Cancer. 2017; 27:668–74.
Article29. Seagle BL, Graves S, Strohl AE, Shahabi S. Survival after primary debulking surgery compared with neoadjuvant chemotherapy in advanced ovarian cancer: a national cancer database study. Int J Gynecol Cancer. 2017; 27:1610–8.30. Siesto G, Cavina R, Romano F, Vitobello D. Primary debulking surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer: a propensity-matched analysis. Am J Clin Oncol. 2018; 41:280–5.31. Taş kın S, Güngör M, Ortaç F, Öztuna D. Neoadjuvant chemotherapy equalizes the optimal cytoreduction rate to primary surgery without improving survival in advanced ovarian cancer. Arch Gynecol Obstet. 2013; 288:1399–403.
Article32. Qin M, Jin Y, Ma L, Zhang YY, Pan LY. The role of neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer: a systematic review and meta-analysis of randomized controlled trials and observational studies. Oncotarget. 2017; 9:8614–28.
Article33. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002; 20:1248–59.
Article34. Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Impact of National Cancer Institute Comprehensive Cancer Centers on ovarian cancer treatment and survival. J Am Coll Surg. 2015; 220:940–50.
Article35. Bristow RE, Chang J, Ziogas A, Randall LM, Anton-Culver H. High-volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014; 132:403–10.
Article36. Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010; 34:433–43.
Article37. Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012; 23(Suppl 10):x111–7.
Article38. Makar AP, Tropé CG, Tummers P, Denys H, Vandecasteele K. Advanced ovarian cancer: primary or interval debulking? Five categories of patients in view of the results of randomized trials and tumor biology: primary debulking surgery and interval debulking surgery for advanced ovarian cancer. Oncologist. 2016; 21:745–54.
Article39. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007; 8:16.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early port-site metastasis during neoadjuvant chemotherapy in advanced stage ovarian cancer: report of two cases
- Neoadjuvant Chemotherapy in Patients with Advanced Epithelial Ovarian Cancer
- Optimal number of neoadjuvant chemotherapy cycles prior to interval debulking surgery in advanced epithelial ovarian cancer: a systematic review and meta-analysis of progression-free survival and overall survival
- Systematic lymph node dissection during interval debulking surgery for advanced epithelial ovarian cancer: a systematic review and meta-analysis
- Negative peritoneal washing cytology during interval debulking surgery predicts overall survival after neoadjuvant chemotherapy for ovarian cancer