Korean J Radiol.
2020 Mar;21(3):316-324. 10.3348/kjr.2019.0647.
Percutaneous Radiofrequency Ablation for Metachronous Hepatic Metastases after Curative Resection of Pancreatic Adenocarcinoma
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jhkimrad@amc.seoul.kr
- KMID: 2470755
- DOI: http://doi.org/10.3348/kjr.2019.0647
Abstract
OBJECTIVE
To retrospectively evaluate the safety and efficacy of percutaneous radiofrequency ablation (RFA) in patients with metachronous hepatic metastases arising from pancreatic adenocarcinoma who had previously received curative surgery.
MATERIALS AND METHODS
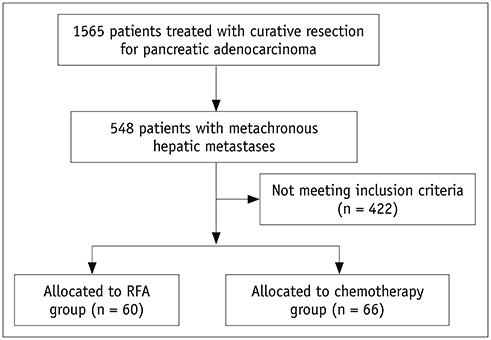
Between 2002 and 2017, percutaneous RFA was performed on 94 metachronous hepatic metastases (median diameter, 1.5 cm) arising from pancreatic cancer in 60 patients (mean age, 60.5 years). Patients were included if they had fewer than five metastases, a maximum tumor diameter of ≤ 5 cm, and disease confined to the liver or stable extrahepatic disease. For comparisons during the same period, we included 66 patients who received chemotherapy only and met the same eligibility criteria described.
RESULTS
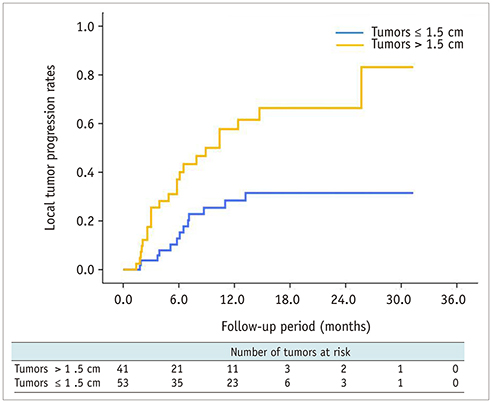
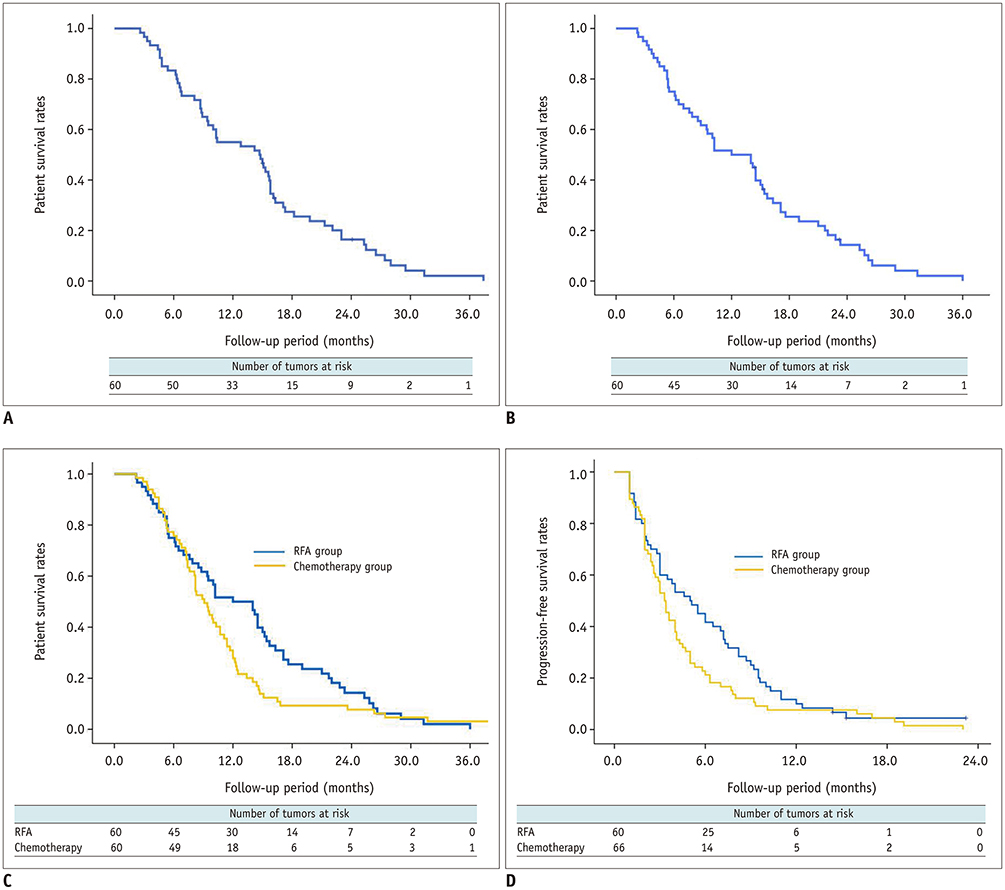
Technical success was achieved in all hepatic metastasis without any procedure-related mortality. During follow-up, local tumor progression of treated lesions was observed in 38.3% of the tumors. Overall median survival and 3-year survival rates were 12 months and 0%, respectively from initial RFA, and 14.7 months and 2.1%, respectively from the first diagnosis of liver metastasis. Multivariate analysis showed that a large tumor diameter of > 1.5 cm, a late TNM stage (≥ IIB) before curative surgery, a time from surgery to recurrence of < 1 year, and the presence of extrahepatic metastasis, were all prognostic of reduced overall survival after RFA. Median overall (12 months vs. 9.1 months, p = 0.094) and progression-free survival (5 months vs. 3.3 months, p = 0.068) were higher in the RFA group than in the chemotherapy group with borderline statistical difference.
CONCLUSION
RFA is safe and may offer successful local tumor control in patients with metachronous hepatic metastases arising from pancreatic adenocarcinoma. Patients with a small diameter tumor, early TNM stage before curative surgery, late hepatic recurrence, and liver-only metastasis benefit most from RFA treatment. RFA provided better survival outcomes than chemotherapy for this specific group with borderline statistical difference.
MeSH Terms
Figure
Reference
-
1. Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg. 1995; 82:111–115.
Article2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30.
Article3. Lockhart AC, Rothenberg ML, Berlin JD. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology. 2005; 128:1642–1654.
Article4. Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999; 189:1–7.5. Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000; 4:567–579.
Article6. Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009; 35:600–604.
Article7. Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990; 66:56–61.
Article8. Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009; 16:836–847.
Article9. Kim JH, Choi EK, Yoon HK, Ko GY, Sung KB, Gwon DI. Transcatheter arterial chemoembolization for hepatic recurrence after curative resection of pancreatic adenocarcinoma. Gut Liver. 2010; 4:384–388.
Article10. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15:2403–2413.
Article11. Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003; 21:3402–3408.
Article12. Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009; 49:453–459.
Article13. Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT Jr. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation--intermediate and long-term survival rates. Radiology. 2009; 253:861–869.
Article14. Park IJ, Kim HC, Yu CS, Kim PN, Won HJ, Kim JC. Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Ann Surg Oncol. 2008; 15:227–232.
Article15. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000; 214:761–768.
Article16. Lee CW, Kim JH, Won HJ, Shin YM, Ko HK, Kim PN, et al. Percutaneous radiofrequency ablation of hepatic metastases from gastric adenocarcinoma after gastrectomy. J Vasc Interv Radiol. 2015; 26:1172–1179.
Article17. Babawale SN, Jensen TM, Frøkjær JB. Long-term survival following radiofrequency ablation of colorectal liver metastases: a retrospective study. World J Gastrointest Surg. 2015; 7:33–38.
Article18. Bai XM, Yang W, Zhang ZY, Jiang AN, Wu W, Lee JC, et al. Long-term outcomes and prognostic analysis of percutaneous radiofrequency ablation in liver metastasis from breast cancer. Int J Hyperthermia. 2019; 35:183–193.
Article19. Park SY, Kim JH, Won HJ, Shin YM, Kim PN. Radiofrequency ablation of hepatic metastases after curative resection of extrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011; 197:W1129–W1134.
Article20. Thomas KT, Bream PR Jr, Berlin J, Meranze SG, Wright JK, Chari RS. Use of percutaneous drainage to treat hepatic abscess after radiofrequency ablation of metastatic pancreatic adenocarcinoma. Am Surg. 2004; 70:496–499.21. Park JB, Kim YH, Kim J, Chang HM, Kim TW, Kim SC, et al. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol. 2012; 23:635–641.
Article22. Hua YQ, Wang P, Zhu XY, Shen YH, Wang K, Shi WD, et al. Radiofrequency ablation for hepatic oligometastatic pancreatic cancer: an analysis of safety and efficacy. Pancreatology. 2017; 17:967–973.
Article23. Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012; 265:958–968.
Article24. Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes--a 10-year experience at a single center. Radiology. 2016; 278:601–611.
Article25. Yoon HM, Kim JH, Shin YM, Won HJ, Kim PN. Percutaneous radiofrequency ablation using internally cooled wet electrodes for treatment of colorectal liver metastases. Clin Radiol. 2012; 67:122–127.
Article26. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014; 25:1691–1705.e4.
Article27. Saad ED, Katz A. Progression-free survival and time to progression as primary end points in advanced breast cancer: often used, sometimes loosely defined. Ann Oncol. 2009; 20:460–464.
Article28. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999; 230:309–318.29. Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997; 21:195–200.
Article30. Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006; 10:511–518.
Article31. Katsumata K, Tomioka H, Sumi T, Yamasaki T, Takagi M, Kato F, et al. Liver metastasis of pancreatic cancer managed by intra-arterial infusion chemotherapy combined with degradable starch microspheres. Int J Clin Oncol. 2003; 8:110–112.
Article32. Kandel P, Wallace MB, Stauffer J, Bolan C, Raimondo M, Woodward TA, et al. Survival of patients with oligometastatic pancreatic ductal adenocarcinoma treated with combined modality treatment including surgical resection: a pilot study. J Pancreat Cancer. 2018; 4:88–94.
Article33. Gleisner AL, Assumpcao L, Cameron JL, Wolfgang CL, Choti MA, Herman JM, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified. Cancer. 2007; 110:2484–2492.
Article34. Lee BC, Lee HG, Park IJ, Kim SY, Kim KH, Lee JH, et al. The role of radiofrequency ablation for treatment of metachronous isolated hepatic metastasis from colorectal cancer. Medicine. 2016; 95:e4999.
Article35. Gbolahan OB, Tong Y, Sehdev A, O'Neil B, Shahda S. Overall survival of patients with recurrent pancreatic cancer treated with systemic therapy: a retrospective study. BMC Cancer. 2019; 19:468.
Article36. Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007; 14:2319–2329.
Article37. De Jong MC, Farnell MB, Sclabas G, Cunningham SC, Cameron JL, Geschwind JF, et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: a dual-center analysis. Ann Surg. 2010; 252:142–148.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Comparison of Hepatic Resection and Radiofrequency Ablation of Hepatic Metastases from Colorectal Cancer
- Comparison of long-term oncologic outcomes between radiofrequency ablation and surgical resection for metachronous isolated hepatic metastases from colorectal cancer
- Liver Metastases in Colorectal Cancer
- Transcatheter Arterial Chemoembolization for Hepatic Recurrence after Curative Resection of Pancreatic Adenocarcinoma
- Is percutaneous destruction of a solitary liver colorectal metastasis as effective as a resection?