Korean J Radiol.
2017 Feb;18(1):162-172. 10.3348/kjr.2017.18.1.162.
Diffusion-Weighted MR Enterography to Monitor Bowel Inflammation after Medical Therapy in Crohn's Disease: A Prospective Longitudinal Study
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea. parksh.radiology@gmail.com
- 2Department of Gastroenterology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea.
- 3Department of Radiology and Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul 03722, Korea.
- KMID: 2468130
- DOI: http://doi.org/10.3348/kjr.2017.18.1.162
Abstract
OBJECTIVE
To prospectively evaluate the performance of diffusion-weighted imaging (DWI) to monitor bowel inflammation after medical therapy for Crohn's disease (CD).
MATERIALS AND METHODS
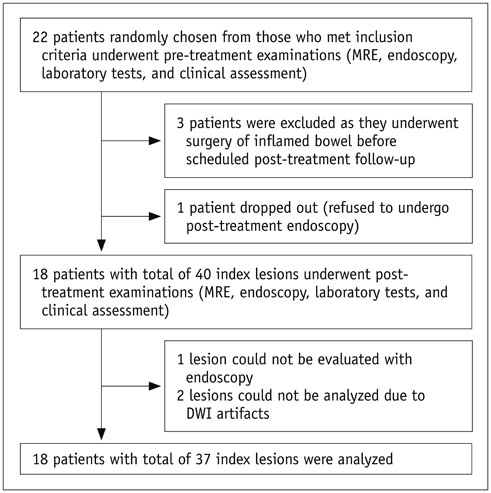
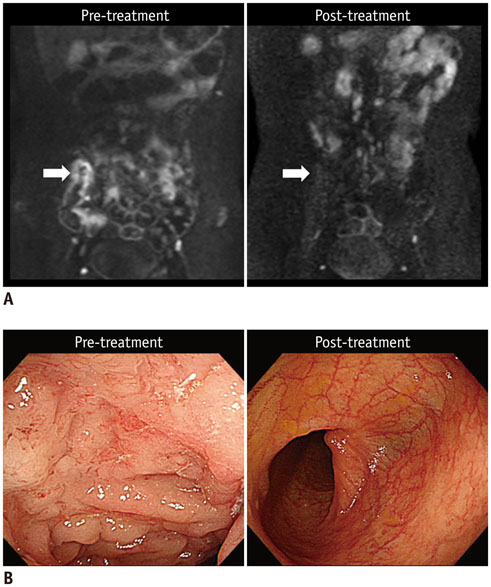
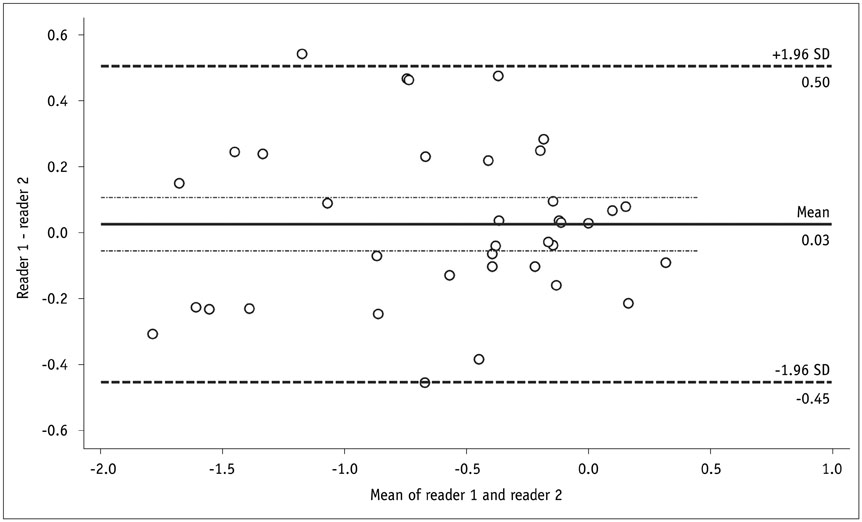
Before and following 1-2 years of medical therapy, between October 2012 and May 2015, 18 randomly selected adult CD patients (male:female, 13:5; mean age ± SD, 25.8 ± 7.9 years at the time of enrollment) prospectively underwent MR enterography (MRE) including DWI (b = 900 s/mm²) and ileocolonoscopy. Thirty-seven prospectively defined index lesions (one contiguous endoscopy-confirmed inflamed area chosen from each inflamed anatomical bowel segment; 1-4 index lesions per patient; median, 2 lesions) were assessed on pre- and post-treatment MRE and endoscopy. Visual assessment of treatment responses on DWI in 4 categories including complete remission and reduced, unchanged or increased inflammation, and measurements of changes in apparent diffusion coefficient (ΔADC), i.e., pre-treatment-post-treatment, were performed by 2 independent readers. Endoscopic findings and CD MRI activity index (CDMI) obtained using conventional MRE served as reference standards.
RESULTS
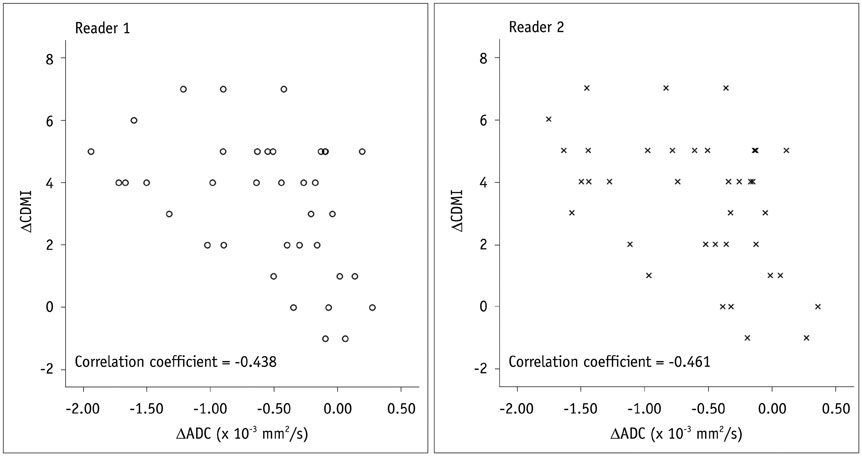
ΔADC significantly differed between improved (i.e., complete remission and reduced inflammation) and unimproved (i.e., unchanged or increased inflammation) lesions: mean ± SD (× 10⻳ mm²/s) of -0.65 ± 0.58 vs. 0.06 ± 0.15 for reader 1 (p = 0.022) and -0.68 ± 0.56 vs. 0.10 ± 0.26 for reader 2 (p = 0.025). DWI accuracy for diagnosing complete remission or improved inflammation ranged from 76% (28/37) to 84% (31/37). A significant negative correlation was noted between ΔADC and ΔCDMI for both readers with correlation coefficients of -0.438 and -0.461, respectively (p < 0.05).
CONCLUSION
DWI is potentially a feasible tool to monitor quantitatively and qualitatively bowel inflammation of CD after medical treatment.
Keyword
MeSH Terms
-
Adolescent
Adult
Anti-Inflammatory Agents, Non-Steroidal/therapeutic use
Crohn Disease/diagnostic imaging/drug therapy/*pathology
*Diffusion Magnetic Resonance Imaging
Endoscopy, Gastrointestinal
Female
Humans
Immunosuppressive Agents/therapeutic use
Inflammation/diagnostic imaging/pathology
Infliximab/therapeutic use
Intestine, Large/diagnostic imaging/pathology
Longitudinal Studies
Male
Prospective Studies
Treatment Outcome
Young Adult
Anti-Inflammatory Agents, Non-Steroidal
Immunosuppressive Agents
Infliximab
Figure
Cited by 2 articles
-
Comparison of Three Magnetization Transfer Ratio Parameters for Assessment of Intestinal Fibrosis in Patients with Crohn's Disease
Jixin Meng, Siyun Huang, CanHui Sun, Zhong-wei Zhang, Ren Mao, Yan-hong Yang, Shi-Ting Feng, Zi-ping Li, XueHua Li
Korean J Radiol. 2020;21(3):290-297. doi: 10.3348/kjr.2019.0217.Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.
Reference
-
1. Choi SH, Kim KW, Lee JY, Kim KJ, Park SH. Diffusion-weighted magnetic resonance enterography for evaluating bowel inflammation in Crohn's disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2016; 22:669–679.2. Park SH. DWI at MR enterography for evaluating bowel inflammation in Crohn disease. AJR Am J Roentgenol. 2016; 207:40–48.3. Cheifetz AS. Management of active Crohn disease. JAMA. 2013; 309:2150–2158.4. Lichtenstein GR, Hanauer SB, Sandborn WJ. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104:465–483. quiz 464, 484.5. Ordás I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn's disease: time for a change. Gut. 2011; 60:1754–1763.6. Sadowski DC, Bernstein CN, Bitton A, Croitoru K, Fedorak RN, Griffiths A. CAG Crohn's Consensus Group. Canadian Association of Gastroenterology Clinical Practice Guidelines: the use of tumour necrosis factor-alpha antagonist therapy in Crohn's disease. Can J Gastroenterol. 2009; 23:185–202.7. Seo N, Park SH, Kim KJ, Kang BK, Lee Y, Yang SK, et al. MR enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology. 2016; 278:762–772.8. Buisson A, Hordonneau C, Goutte M, Boyer L, Pereira B, Bommelaer G. Diffusion-weighted magnetic resonance imaging is effective to detect ileocolonic ulcerations in Crohn's disease. Aliment Pharmacol Ther. 2015; 42:452–460.9. Tielbeek JA, Ziech ML, Li Z, Lavini C, Bipat S, Bemelman WA, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn's disease assessment with histopathology of surgical specimens. Eur Radiol. 2014; 24:619–629.10. Hordonneau C, Buisson A, Scanzi J, Goutorbe F, Pereira B, Borderon C, et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn's disease: validation of quantitative index of activity. Am J Gastroenterol. 2014; 109:89–98.11. Caruso A, D'Incà R, Scarpa M, Manfrin P, Rudatis M, Pozza A, et al. Diffusion-weighted magnetic resonance for assessing ileal Crohn's disease activity. Inflamm Bowel Dis. 2014; 20:1575–1583.12. Ream JM, Dillman JR, Adler J, Khalatbari S, McHugh JB, Strouse PJ, et al. MRI diffusion-weighted imaging (DWI) in pediatric small bowel Crohn disease: correlation with MRI findings of active bowel wall inflammation. Pediatr Radiol. 2013; 43:1077–1085.13. Neubauer H, Pabst T, Dick A, Machann W, Evangelista L, Wirth C, et al. Small-bowel MRI in children and young adults with Crohn disease: retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr Radiol. 2013; 43:103–114.14. Buisson A, Joubert A, Montoriol PF, Da Ines D, Hordonneau C, Pereira B, et al. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn's disease. Aliment Pharmacol Ther. 2013; 37:537–545.15. Bhatnagar G, Dikaios N, Prezzi D, Vega R, Halligan S, Taylor SA. Changes in dynamic contrast-enhanced pharmacokinetic and diffusion-weighted imaging parameters reflect response to anti-TNF therapy in Crohn's disease. Br J Radiol. 2015; 88:20150547.16. Baars JE, Nuij VJ, Oldenburg B, Kuipers EJ, van der Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis. 2012; 18:1634–1640.17. Peyrin-Biroulet L, Reinisch W, Colombel JF, Mantzaris GJ, Kornbluth A, Diamond R, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut. 2014; 63:88–95.18. Catalano OA, Gee MS, Nicolai E, Selvaggi F, Pellino G, Cuocolo A, et al. Evaluation of quantitative PET/MR enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease. Radiology. 2016; 278:792–800.19. Kovanlikaya A, Beneck D, Rose M, Renjen P, Dunning A, Solomon A, et al. Quantitative apparent diffusion coefficient (ADC) values as an imaging biomarker for fibrosis in pediatric Crohn's disease: preliminary experience. Abdom Imaging. 2015; 40:1068–1074.20. Adler J, Punglia DR, Dillman JR, Polydorides AD, Dave M, Al-Hawary MM, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn's disease. Inflamm Bowel Dis. 2012; 18:849–856.21. Zappa M, Stefanescu C, Cazals-Hatem D, Bretagnol F, Deschamps L, Attar A, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn's disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011; 17:984–993.22. Kim KJ, Lee Y, Park SH, Kang BK, Seo N, Yang SK, et al. Diffusion-weighted MR enterography for evaluating Crohn's disease: how does it add diagnostically to conventional MR enterography? Inflamm Bowel Dis. 2015; 21:101–109.23. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989; 170:2–6. discussion 16-19.24. Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second european evidence-based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis. 2010; 4:7–27.25. Colombel JF, Rutgeerts PJ, Sandborn WJ, Yang M, Camez A, Pollack PF, et al. Adalimumab induces deep remission in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014; 12:414–422.e5.26. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.27. Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012; 142:1102–1111.e2.28. Mary JY, Modigliani R. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Gut. 1989; 30:983–989.29. Steward MJ, Punwani S, Proctor I, Adjei-Gyamfi Y, Chatterjee F, Bloom S, et al. Non-perforating small bowel Crohn's disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012; 81:2080–2088.30. Tielbeek JA, Makanyanga JC, Bipat S, Pendsé DA, Nio CY, Vos FM, et al. Grading Crohn disease activity with MRI: interobserver variability of MRI features, MRI scoring of severity, and correlation with Crohn disease endoscopic index of severity. AJR Am J Roentgenol. 2013; 201:1220–1228.31. Yang Z, Zhou M. Weighted kappa statistic for clustered matched-pair ordinal data. Comput Stat Data Anal. 2015; 82:1–18.32. Kim SY, Park SH. Reply to what is the role of diffusion-weighted imaging in ileocolonic Crohn's disease? Inflamm Bowel Dis. 2015; 21:E9–E10.33. Bilgili MY. Reproductibility of apparent diffusion coefficients measurements in diffusion-weighted MRI of the abdomen with different b values. Eur J Radiol. 2012; 81:2066–2068.34. Braithwaite AC, Dale BM, Boll DT, Merkle EM. Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology. 2009; 250:459–465.35. Kim SY, Lee SS, Byun JH, Park SH, Kim JK, Park B, et al. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology. 2010; 255:815–823.36. Kim SY, Lee SS, Park B, Kim N, Kim JK, Park SH, et al. Reproducibility of measurement of apparent diffusion coefficients of malignant hepatic tumors: effect of DWI techniques and calculation methods. J Magn Reson Imaging. 2012; 36:1131–1138.37. Miquel ME, Scott AD, Macdougall ND, Boubertakh R, Bharwani N, Rockall AG. In vitro and in vivo repeatability of abdominal diffusion-weighted MRI. Br J Radiol. 2012; 85:1507–1512.38. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010; 254:47–66.39. Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990; 43:543–549.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Computed Tomography Enterography/Magnetic Resonance Enterography: Is It in Prime Time?
- Magnetic resonance enterography for the evaluation of the deep small intestine in Crohn's disease
- A Look into the Small Bowel in Crohn's Disease
- Computed Tomography Enterography and Magnetic Resonance Enterography in the Diagnosis of Crohn's Disease
- Comprehensive Review of Magnetic Resonance Enterography-Based Activity Scoring Systems for Crohn’s Disease