Transl Clin Pharmacol.
2019 Dec;27(4):149-154. 10.12793/tcp.2019.27.4.149.
A review of three years' experience of the first pharmacometrics company in Korea
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, The Catholic University of Korea, Seoul 06591, Republic of Korea.
- 2Q-fitter, Inc., Seoul 06199, Republic of Korea. si.jeon@qfitter.com
- KMID: 2467969
- DOI: http://doi.org/10.12793/tcp.2019.27.4.149
Abstract
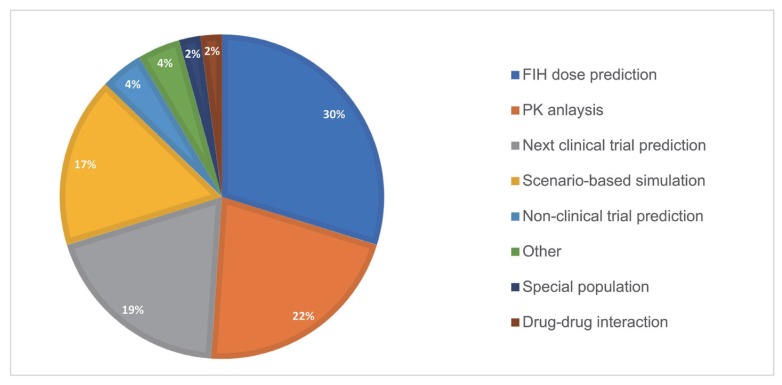
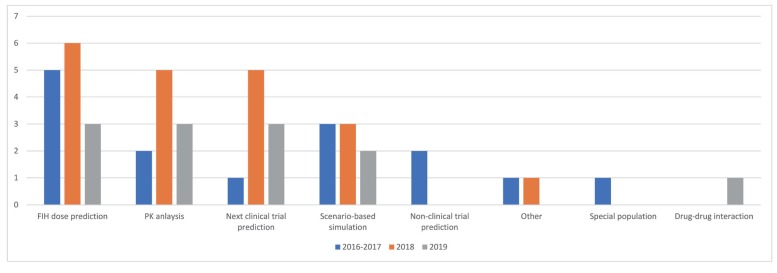
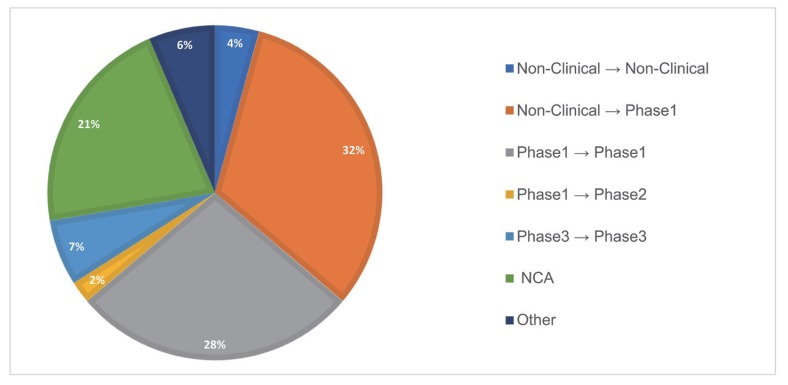
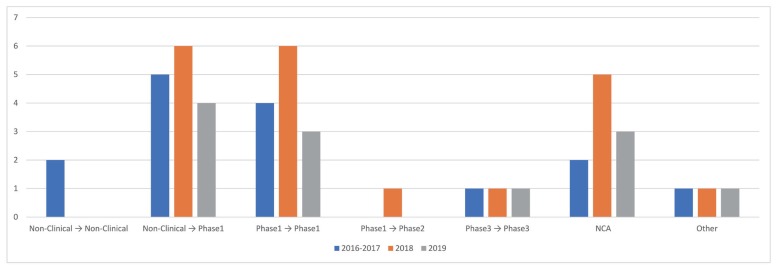
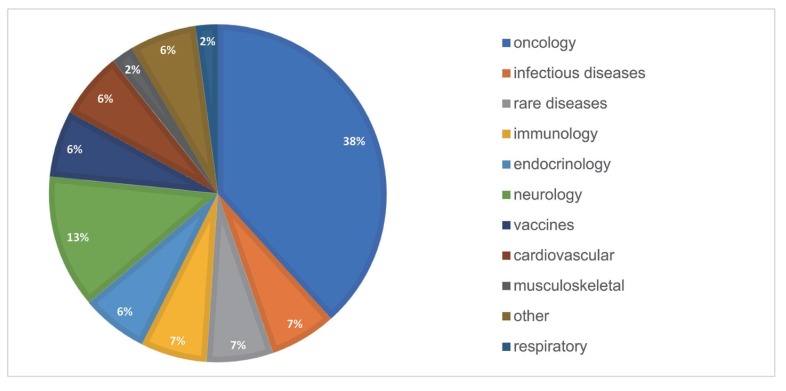
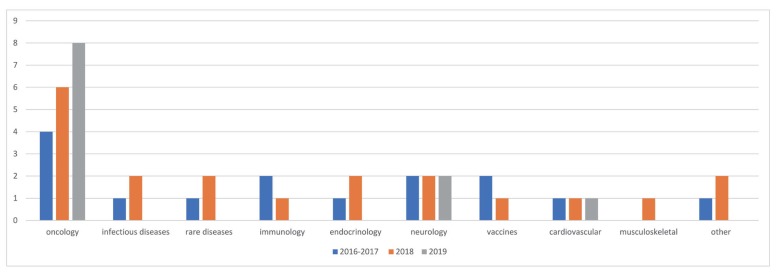
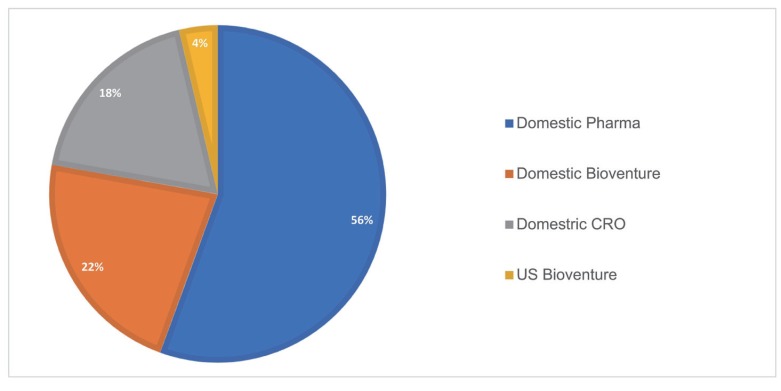
- As the pharmaceutical industry in Korea is reaching the golden era of drug discovery due to increased investments in research and development and government funds, the need for a more efficient tool for the quantitative analysis has emerged. Therefore, the demand for pharmacometrics (PMx) consultancy services increased. Higher quality service suitable for regulatory submission and out-licensing deals were desired. In this analysis, we compiled and summarized 3 years of experiences of Q-fitter, the first PMx consultancy service company providing PMx analysis to the pharmaceutical industry in Korea. The projects were organized by companies, company types, indications, therapeutic areas, drug development stages, purposes, and scope of services. Within each category, we subcategorized the sections and assessed proportions and a year-over-year trend. As a result, we observed an increase in the number of projects in an average of ~170% per year, with the most frequent types of companies collaborated being the domestic pharmaceutical companies. Among the projects, ~72% involved modeling and simulation using population pharmacokinetic (PK) models, and the other included non-compartmental analysis (NCA), drug-drug interaction (DDI) prediction, and interpretation of the modeling results. The most sought-after purpose in PMx analysis was first-in-human (FIH) dose prediction followed by PK analysis, next clinical trial prediction, and scenario-based simulation. Oncology has been the top therapeutic area of interest every year consisting of ~38% of total projects, followed by Neurology (~13%). From this review, we were able to characterize the PMx service needs and spot the trend of current PMx practices in Korea.
Keyword
Figure
Reference
-
1. Gobburu JV. Pharmacometrics 2020. J Clin Pharmacol. 2010; 50(9 Suppl):151S–157S. DOI: 10.1177/0091270010376977. PMID: 20881229.
Article2. Schuck E, Bohnert T, Chakravarty A, Damian-Iordache V, Gibson C, Hsu CP, et al. Preclinical pharmacokinetic/pharmacodynamic modeling and simulation in the pharmaceutical industry: an IQ consortium survey examining the current landscape. AAPS J. 2015; 17:462–473. DOI: 10.1208/s12248-014-9716-2. PMID: 25630504.
Article3. Chein JY, Friedrich S, Heathman MA, de Alwis DP, Sinha V. Pharmacokinetics/pharmacodynamics and the stages of drug development: role of modeling and simulation. AAPS J. 2005; 7:E544–E559. DOI: 10.1208/aapsj070355. PMID: 16353932.
Article4. Lee M, Choi M. Analysis on time-lag effect of research and development investment in the pharmaceutical industry in Korea. Osong Public Health Res Perspect. 2015; 6:241–248. DOI: 10.1016/j.phrp.2015.07.001. PMID: 26473091.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Evaluation of Food Delivery Worker Sanitation Management Practices that Supply Food to School Foodservices
- Pharmacometric models simulation using NONMEM, Berkeley Madonna and R
- Effect of Job Insecurity on Job related Depression and Anxiety: Large- and Small-sized Company Employees
- A Study on Brand Awareness of Contract Foodservice Management Company in Incheon Area
- Tips for the choice of initial estimates in NONMEM