J Korean Assoc Oral Maxillofac Surg.
2019 Dec;45(6):332-342. 10.5125/jkaoms.2019.45.6.332.
Socket preservation using eggshell-derived nanohydroxyapatite with platelet-rich fibrin as a barrier membrane: a new technique
- Affiliations
-
- 1Department of Oral and Maxillofacia Surgery, Sibar Institute of Dental Sciences, Guntur, India. drvivekanandsk@gmail.com
- 2Department of Physics, Periyar University Salem, Salem, India.
- 3Department of Oral and Maxillofacial Pathology, Sibar Institute of Dental Sciences, Guntur, India.
- KMID: 2467185
- DOI: http://doi.org/10.5125/jkaoms.2019.45.6.332
Abstract
OBJECTIVES
Socket grafting is vital to prevent bone resorption after tooth extraction. Several techniques to prevent resorption have been described, and various bone graft substitutes have been developed and used with varying success. We conducted this pilot study to evaluate the performance of nanohydroxyapatite (nHA) derived from chicken eggshells in socket preservation.
MATERIALS AND METHODS
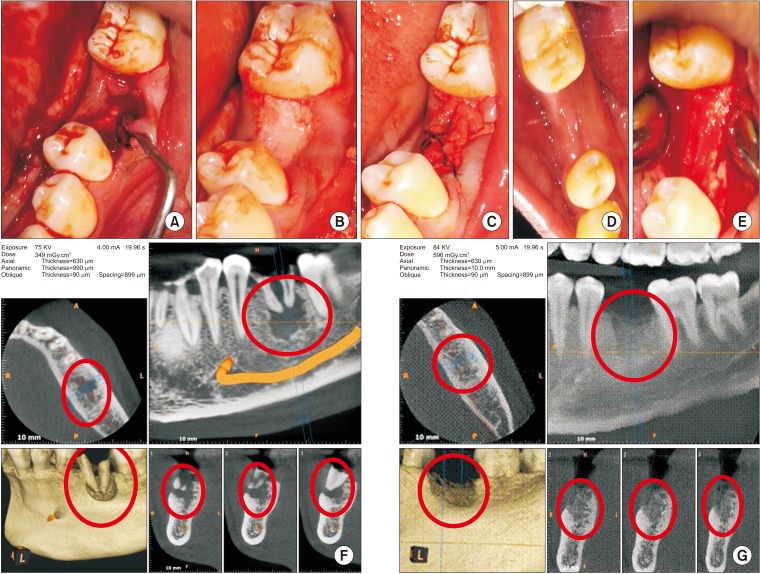
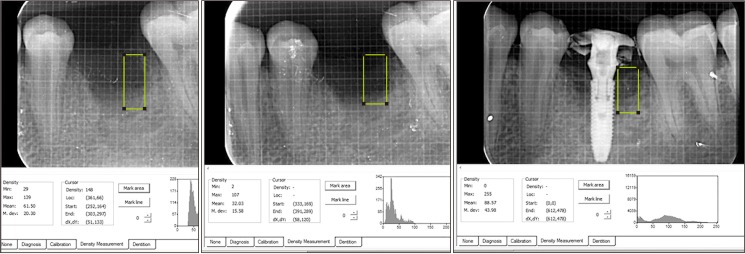
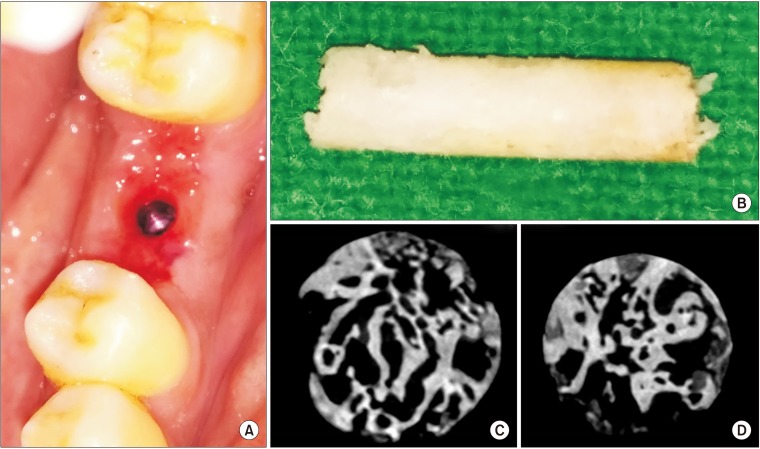
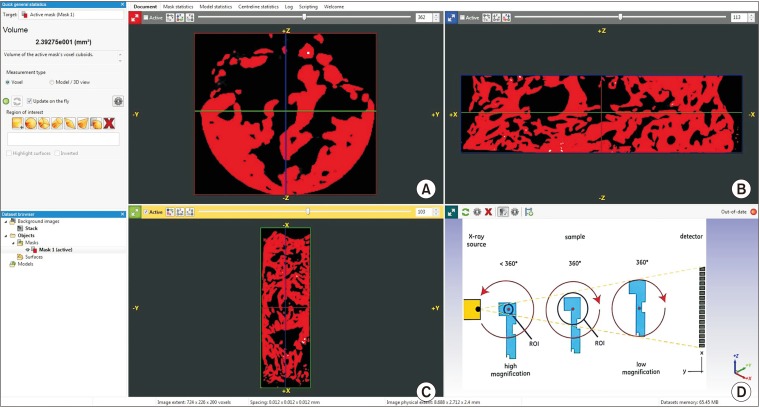
This was a prospective, single center, outcome assessor-blinded evaluation of 23 sockets (11 patients) grafted with nHA and covered with platelet-rich fibrin (PRF) membrane as a barrier. Bone width and radiographic bone density were measured using digital radiographs at 1, 12, and 24 weeks post-procedure. Postoperative histomorphometric and micro-computed tomography (CT) evaluation were performed. The study protocol was approved by the Institutional Ethics Committee.
RESULTS
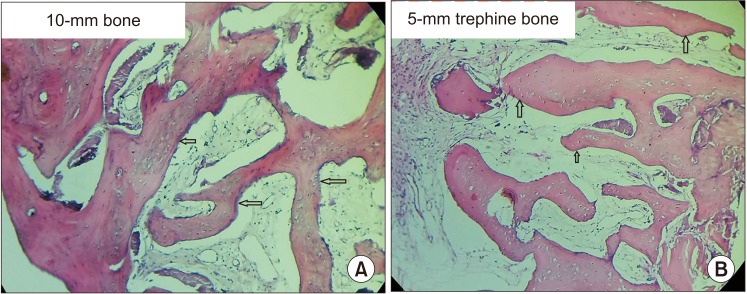
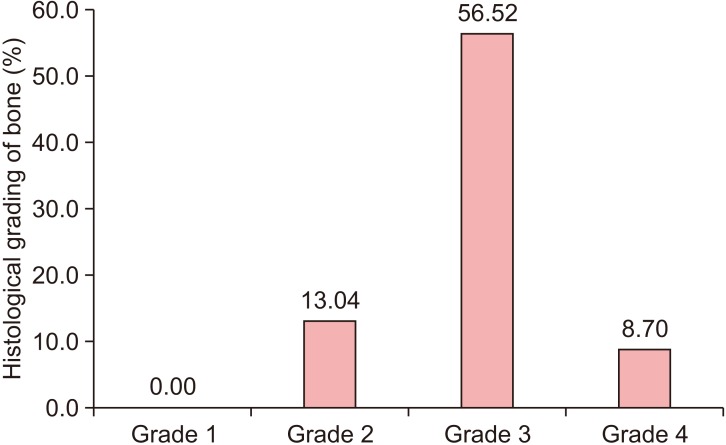
All patients had uneventful wound healing without graft material displacement or leaching despite partial exposure of the grafted socket. Tissue re-epithelialized with thick gingival biotype (>3 mm). Width of the bone was maintained and radiographic density increased significantly with a trabecular pattern (73.91% of sockets) within 12 weeks. Histomorphometric analysis showed 56.52% Grade 3 bone formation and micro-CT analysis revealed newly formed bone with interconnecting trabeculae.
CONCLUSION
Use of a PRF membrane with nHA resulted in good bone regeneration in sockets. Use of a PRF membrane prevents periosteal-releasing incisions for primary closure, thereby facilitating the preservation of keratinized mucosa and gingival architecture. This technique, which uses eggshell-derived nHA and PRF membrane from the patient's own blood, is innovative and is free of disease transfer risks. nHA is a promising economic bone graft substitute for bone regeneration and reconstruction because of the abundant availability of eggshell waste as a raw material.
MeSH Terms
Figure
Reference
-
1. Atieh MA, Alsabeeha NH, Payne AG, Duncan W, Faggion CM, Esposito M. Interventions for replacing missing teeth: alveolar ridge preservation techniques for dental implant site development. Cochrane Database Syst Rev. 2015; (5):CD010176. PMID: 26020735.
Article2. Camargo PM, Lekovic V, Weinlaender M, Klokkevold PR, Kenney EB, Dimitrijevic B, et al. Influence of bioactive glass on changes in alveolar process dimensions after exodontia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 90:581–586. PMID: 11077380.
Article3. Iasella JM, Greenwell H, Miller RL, Hill M, Drisko C, Bohra AA, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003; 74:990–999. PMID: 12931761.
Article4. Lekovic V, Kenney EB, Weinlaender M, Han T, Klokkevold P, Nedic M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997; 68:563–570. PMID: 9203100.
Article5. Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, Dimitrijevic B, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998; 69:1044–1049. PMID: 9776033.
Article6. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003; 23:313–323. PMID: 12956475.7. Tan WL, Wong TL, Wong MC, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012; 23 Suppl 5:1–21.
Article8. John V, De Poi R, Blanchard S. Socket preservation as a precursor of future implant placement: review of the literature and case reports. Compend Contin Educ Dent. 2007; 28:646–653. quiz 654, 671. PMID: 18186170.9. Ten Heggeler JM, Slot DE, Van der Weijden GA. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: a systematic review. Clin Oral Implants Res. 2011; 22:779–788. PMID: 21091540.
Article10. Hämmerle CH, Araújo MG, Simion M. Osteology Consensus Group 2011. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin Oral Implants Res. 2012; 23 Suppl 5:80–82. PMID: 22211307.
Article11. Vignoletti F, Matesanz P, Rodrigo D, Figuero E, Martin C, Sanz M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin Oral Implants Res. 2012; 23 Suppl 5:22–38.
Article12. Vittorini Orgeas G, Clementini M, De Risi V, de Sanctis M. Surgical techniques for alveolar socket preservation: a systematic review. Int J Oral Maxillofac Implants. 2013; 28:1049–1061. PMID: 23869363.
Article13. Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991; 2:187–208. PMID: 10149083.
Article14. Habal MB. Different forms of bone grafts. In : Habal MB, Reddi AH, Mitchell J, editors. Bone grafts and bone substitutes. Philadelphia: W.B. Saunders;1992. p. 6–8.15. Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991; 74:1487–1510.
Article16. Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. American Academy of Orthopaedic Surgeons. The Committee on Biological Implants. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am. 2001; 83-A Suppl 2 Pt 2:98–103.17. SureshKumar G, Thamizhavel A, Girija EK. Microwave conversion of eggshells into flower-like hydroxyapatite nanostructure for biomedical applications. Mater Lett. 2012; 76:198–200.18. SureshKumar G, Thamizhavel A, Yokogawa Y, NarayanaKalkura S, Girija EK. Synthesis, characterization and in vitro studies of zinc and carbonate co-substituted nano-hydroxyapatite for biomedical applications. Mater Chem Phys. 2012; 134:1127–1135.19. SureshKumar G, Girija EK. Flower-like hydroxyapatite nanostructure obtained from eggshell: a candidate for biomedical applications. Ceram Int. 2013; 39:8293–8299.20. Park JW, Bae SR, Suh JY, Lee DH, Kim SH, Kim H, et al. Evaluation of bone healing with eggshell-derived bone graft substitutes in rat calvaria: a pilot study. J Biomed Mater Res A. 2008; 87:203–214. PMID: 18085653.
Article21. Kim SH, Kim W, Cho JH, Oh NS, Lee MH, Lee SJ. Comparison of bone formation in rabbits using hydroxyapatite and β-tricalcium phosphate scaffolds fabricated from egg shells. Adv Mater Res. 2008; 47-50:999–1002.
Article22. Lee SW, Kim SG, Balázsi C, Chae WS, Lee HO. Comparative study of hydroxyapatite from eggshells and synthetic hydroxyapatite for bone regeneration. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:348–355. PMID: 22676827.
Article23. Kattimani V, Lingamaneni KP, Chakravarthi PS, Kumar TS, Siddharthan A. Eggshell-derived hydroxyapatite: a new era in bone regeneration. J Craniofac Surg. 2016; 27:112–117. PMID: 26674907.24. Kattimani VS, Chakravarthi SP, Neelima Devi KN, Sridhar MS, Prasad LK. Comparative evaluation of bovine derived hydroxyapatite and synthetic hydroxyapatite graft in bone regeneration of human maxillary cystic defects: a clinico-radiological study. Indian J Dent Res. 2014; 25:594–601. PMID: 25511058.
Article25. Kattimani VS, Bajantai NV, Sriram SK, Sriram RR, Rao VK, Desai PD. Observer strategy and radiographic classification of healing after grafting of cystic defects in maxilla: a radiological appraisal. J Contemp Dent Pract. 2013; 14:227–232. PMID: 23811650.26. Brownfield LA, Weltman RL. Ridge preservation with or without an osteoinductive allograft: a clinical, radiographic, micro-computed tomography, and histologic study evaluating dimensional changes and new bone formation of the alveolar ridge. J Periodontol. 2012; 83:581–589. PMID: 21942791.
Article27. Morjaria KR, Wilson R, Palmer RM. Bone healing after tooth extraction with or without an intervention: a systematic review of randomized controlled trials. Clin Implant Dent Relat Res. 2014; 16:1–20. PMID: 22405099.
Article28. Ripamonti U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 1996; 17:31–35. PMID: 8962945.
Article29. Kasaj A, Willershausen B, Reichert C, Röhrig B, Smeets R, Schmidt M. Ability of nanocrystalline hydroxyapatite paste to promote human periodontal ligament cell proliferation. J Oral Sci. 2008; 50:279–285. PMID: 18818463.
Article30. Gosain AK, Song L, Riordan P, Amarante MT, Nagy PG, Wilson CR, et al. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: part I. Plast Reconstr Surg. 2002; 109:619–630. PMID: 11818845.
Article31. Gross JS. Bone grafting materials for dental applications: a practical guide. Compend Contin Educ Dent. 1997; 18:1013–1018. 1020–1022. 1024passim; quiz. PMID: 9533311.32. Darby I. Periodontal materials. Aust Dent J. 2011; 56 Suppl 1:107–118.
Article33. Ashman A. Postextraction ridge preservation using a synthetic alloplast. Implant Dent. 2000; 9:168–176. PMID: 11307396.
Article34. Gholami GA, Najafi B, Mashhadiabbas F, Goetz W, Najafi S. Clinical, histologic and histomorphometric evaluation of socket preservation using a synthetic nanocrystalline hydroxyapatite in comparison with a bovine xenograft: a randomized clinical trial. Clin Oral Implants Res. 2012; 23:1198–1204. PMID: 22092485.
Article35. Götz W, Gerber T, Michel B, Lossdörfer S, Henkel KO, Heinemann F. Immunohistochemical characterization of nanocrystalline hydroxyapatite silica gel (NanoBone(r)) osteogenesis: a study on biopsies from human jaws. Clin Oral Implants Res. 2008; 19:1016–1026. PMID: 18828818.36. Chris Arts JJ, Verdonschot N, Schreurs BW, Buma P. The use of a bioresorbable nano-crystalline hydroxyapatite paste in acetabular bone impaction grafting. Biomaterials. 2006; 27:1110–1118. PMID: 16098583.
Article37. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000; 21:1803–1810. PMID: 10905463.
Article38. Froum S, Orlowski W. Ridge preservation utilizing an alloplast prior to implant placement--clinical and histological case reports. Pract Periodontics Aesthet Dent. 2000; 12:393–402. quiz 404. PMID: 11404863.39. Ashman A, LoPinto J, Rosenlicht J. Ridge augmentation for immediate postextraction implants: eight year retrospective study. Pract Periodontics Aesthet Dent. 1995; 7:85–94. quiz 95. PMID: 7670079.40. Boyne PJ. Use of HTR in tooth extraction sockets to maintain alveolar ridge height and increase concentration of alveolar bone matrix. Gen Dent. 1995; 43:470–473. PMID: 8941742.41. Pagni G, Pellegrini G, Giannobile WV, Rasperini G. Postextraction alveolar ridge preservation: biological basis and treatments. Int J Dent. 2012; 2012:151030. PMID: 22737169.
Article42. Fickl S, Zuhr O, Wachtel H, Bolz W, Huerzeler MB. Hard tissue alterations after socket preservation: an experimental study in the beagle dog. Clin Oral Implants Res. 2008; 19:1111–1118. PMID: 18983313.
Article43. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005; 32:212–218. PMID: 15691354.
Article44. Aimetti M, Romano F, Griga FB, Godio L. Clinical and histologic healing of human extraction sockets filled with calcium sulfate. Int J Oral Maxillofac Implants. 2009; 24:902–909. PMID: 19865631.45. Murphy KG. Postoperative healing complications associated with Gore-Tex periodontal material. Part II. effect of complications on regeneration. Int J Periodontics Restorative Dent. 1995; 15:548–561. PMID: 9601253.46. Murphy KG. Postoperative healing complications associated with Gore-Tex periodontal material. Part I. incidence and characterization. Int J Periodontics Restorative Dent. 1995; 15:363–375. PMID: 8593986.47. De Sanctis M, Zucchelli G, Clauser C. Bacterial colonization of bioabsorbable barrier material and periodontal regeneration. J Periodontol. 1996; 67:1193–1200. PMID: 8959569.48. Miron RJ, Choukroun J. Platelet rich fibrin in regenerative dentistry: biological background and clinical indications. Hoboken: John Wiley & Sons;2017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The double-barrier technique using platelet-rich fibrin for closure of oroantral fistulas
- Regenerative Potential of Platelet Rich Fibrin (PRF) in Socket Preservation in Comparison with Conventional Treatment Modalities: A Systematic Review and Meta-Analysis
- Clinical and Histopathological Study Using Platelet Rich Plasma and Bone Grafts in Extraction Sockets
- Platelet rich fibrin in the management of established dry socket
- Trends in Clinical Application of Platelet-Derived Bioproducts