Investig Clin Urol.
2020 Jan;61(1):19-27. 10.4111/icu.2020.61.1.19.
Enzalutamide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer: A retrospective Korean multicenter study in a real-world setting
- Affiliations
-
- 1Department of Urology, Chonnam National University Medical School, Gwangju, Korea. urokwon@gmail.com
- 2Department of Urology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Urology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Urology, Korea University College of Medicine, Seoul, Korea.
- 5Department of Urology, Center for Prostate Cancer, Korea National Cancer Center, Goyang, Korea.
- 6Department of Urology, Severance Hospital, Yonsei University Health System, Seoul, Korea.
- 7Department of Urology, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea.
- 8Department of Urology, Kyungpook National University School of Medicine, Daegu, Korea.
- 9Department of Urology, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 10Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 11Department of Urology, The Catholic University of Korea, Seoul St. Mary Hospital's, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 12Department of Urology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 13Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2466788
- DOI: http://doi.org/10.4111/icu.2020.61.1.19
Abstract
- PURPOSE
This study aimed to evaluate the clinical efficacy of enzalutamide in chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC) patients using real-world data from Korean patients.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 199 chemotherapy-naïve patients with mCRPC at 13 tertiary centers in Korea between 2014 and 2017. All patients received enzalutamide daily and 89 patients received concurrent androgen deprivation therapy (ADT).
RESULTS
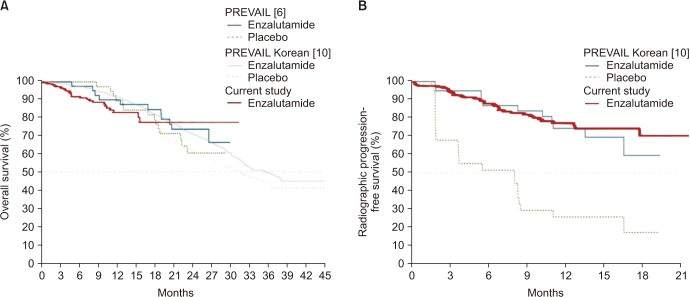
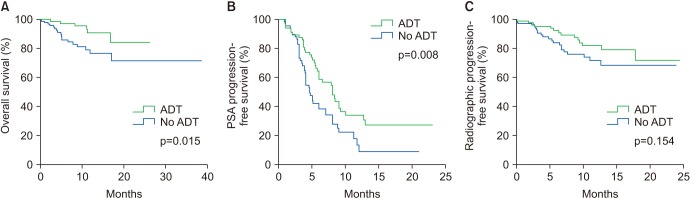
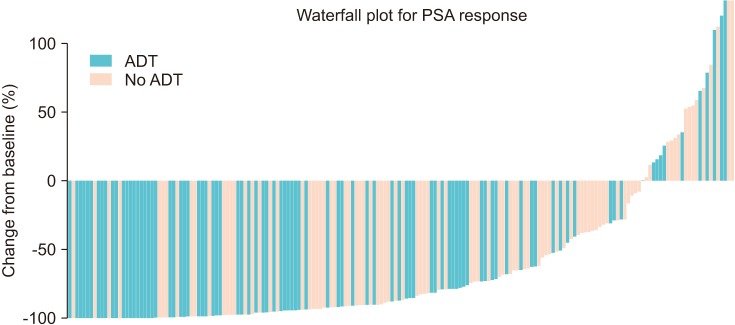
The median age of the patients was 74 years. Initial results showed that 81.5% of the patients had Gleason score ≥8 and 33.3% of the patients had European Cooperative Oncology Group Performance Status 0. The overall mortality rate was 12%. The median OS was not archieved and 76.7% of patients were alive at 30 months. Median time until PSA progression was 6 months. The overall survival rate at 2 years was significantly higher (84.6% vs. 71.7%, p=0.015) and the duration of PSA progression-free survival was significantly longer (8.0 vs. 4.6 months, p=0.008) in patients receiving concurrent ADT than in those receiving enzalutamide alone. The incidence of adverse events of grade 3 or higher was 1.7%. Multivariate Cox proportional hazard analysis indicated that ADT administered concurrently with enzalutamide significantly improved the overall survival (hazard ratio, 0.346; 95% confidence interval, 0.125-0.958).
CONCLUSIONS
Enzalutamide is effective and safe for chemotherapy-naïve patients with mCRPC. Furthermore, the overall survival was significantly higher in patients receiving enzalutamide and concurrent ADT than in patients receiving enzalutamide alone.
MeSH Terms
Figure
Cited by 1 articles
-
Oncological Outcomes in Men with Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide with versus without Confirmatory Bone Scan
Chang Wook Jeong, Jang Hee Han, Dong Deuk Kwon, Jae Young Joung, Choung-Soo Kim, Hanjong Ahn, Jun Hyuk Hong, Tae-Hwan Kim, Byung Ha Chung, Seong Soo Jeon, Minyong Kang, Sung Kyu Hong, Tae Young Jung, Sung Woo Park, Seok Joong Yun, Ji Yeol Lee, Seung Hwan Lee, Seok Ho Kang, Cheol Kwak
Cancer Res Treat. 2024;56(2):634-641. doi: 10.4143/crt.2023.848.
Reference
-
1. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141. PMID: 25761484.
Article2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.
Article3. Lee DH, Jung HB, Chung MS, Lee SH, Chung BH. The change of prostate cancer treatment in Korea: 5 year analysis of a single institution. Yonsei Med J. 2013; 54:87–91. PMID: 23225803.
Article4. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008; 26:1148–1159. PMID: 18309951.
Article5. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351:1502–1512. PMID: 15470213.
Article6. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371:424–433. PMID: 24881730.
Article7. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012; 367:1187–1197. PMID: 22894553.
Article8. Saad F, de Bono J, Shore N, Fizazi K, Loriot Y, Hirmand M, et al. Efficacy outcomes by baseline prostate-specific antigen quartile in the AFFIRM trial. Eur Urol. 2015; 67:223–230. PMID: 25171902.
Article9. Loriot Y, Miller K, Sternberg CN, Fizazi K, De Bono JS, Chowdhury S, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015; 16:509–521. PMID: 25888263.
Article10. Kim CS, Theeuwes A, Kwon DD, Choi YD, Chung BH, Lee HM, et al. The PREVAIL trial of enzalutamide in men with chemotherapy-naïve, metastatic castration-resistant prostate cancer: post hoc analysis of Korean patients. Investig Clin Urol. 2016; 57:174–183.
Article11. Merseburger AS, Hammerer P, Rozet F, Roumeguère T, Caffo O, da Silva FC, et al. Androgen deprivation therapy in castrate-resistant prostate cancer: how important is GnRH agonist backbone therapy. World J Urol. 2015; 33:1079–1085. PMID: 25261259.
Article12. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. European Association of Urology. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014; 65:467–479. PMID: 24321502.
Article13. Merseburger AS, Björk T, Whitehouse J, Meani D. Treatment costs for advanced prostate cancer using luteinizing hormone-releasing hormone agonists: a solid biodegradable leuprorelin implant versus other formulations. J Comp Eff Res. 2015; 4:447–453. PMID: 25521079.
Article14. Taylor CD, Elson P, Trump DL. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993; 11:2167–2172. PMID: 8229130.
Article15. Hussain M, Wolf M, Marshall E, Crawford ED, Eisenberger M. Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: a Southwest Oncology Group report. J Clin Oncol. 1994; 12:1868–1875. PMID: 8083710.
Article16. Lee JL, Eun Kim J, Ahn JH, Lee DH, Lee J, Kim CS, et al. Role of androgen deprivation treatment in patients with castration-resistant prostate cancer, receiving docetaxel-based chemotherapy. Am J Clin Oncol. 2011; 34:140–144. PMID: 20686407.
Article17. Bong GW, Clarke HS Jr, Hancock WC, Keane TE. Serum testosterone recovery after cessation of long-term luteinizing hormone-releasing hormone agonist in patients with prostate cancer. Urology. 2008; 71:1177–1180. PMID: 18279929.
Article18. Kobayashi T, Nishizawa K, Mitsumori K. Individual variation of hormonal recovery after cessation of luteinizing hormone-releasing hormone agonist therapy in men receiving long-term medical castration therapy for prostate cancer. Scand J Urol Nephrol. 2006; 40:198–203. PMID: 16809259.
Article19. Kaku H, Saika T, Tsushima T, Ebara S, Senoh T, Yamato T, et al. Time course of serum testosterone and luteinizing hormone levels after cessation of long-term luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Prostate. 2006; 66:439–444. PMID: 16329145.
Article20. Hall MC, Fritzsch RJ, Sagalowsky AI, Ahrens A, Petty B, Roehrborn CG. Prospective determination of the hormonal response after cessation of luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Urology. 1999; 53:898–902. discussion 902–3. PMID: 10223480.
Article21. Pinski J, Xiong S, Wang Q, Stanczyk F, Hawes D, Liu SV. Effect of luteinizing hormone on the steroidogenic pathway in prostate cancer. Prostate. 2011; 71:892–898. PMID: 21456071.
Article22. O'Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004; 90:2317–2325. PMID: 15150570.23. Ohlmann CH, Jäschke M, Jaehnig P, Krege S, Gschwend J, Rexer H, et al. Abiraterone acetate plus LHRH therapy versus abiraterone acetate while sparing LHRH therapy in patients with progressive, metastatic and chemotherapy-naïve, castration-resistant prostate cancer (SPARE): study protocol for a randomized controlled trial. Trials. 2017; 18:457. PMID: 28978327.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Serum Testosterone Level as Possible Predictive Marker for Prognosis in Metastatic Castration-Resistant Prostate Cancer Patients Treated With Enzalutamide
- The Current Status of Metastatic Castration-Naïve Prostate Cancer Management
- Hormonal therapy and chemotherapy for advanced prostate cancer
- Systemic Therapies for Metastatic Castration-Resistant Prostate Cancer: An Updated Review