Anat Cell Biol.
2019 Dec;52(4):378-384. 10.5115/acb.19.191.
The effects of head rotation and tilt on oral pressure and muscle activity
- Affiliations
-
- 1Department of Occupational Therapy, Division of Health Science, Dongseo University, Busan, Korea.
- 2Department of Dental Hygiene, Division of Health Sciences, Dongseo University, Busan, Korea. dahye1124@dongseo.ac.kr
- KMID: 2466689
- DOI: http://doi.org/10.5115/acb.19.191
Abstract
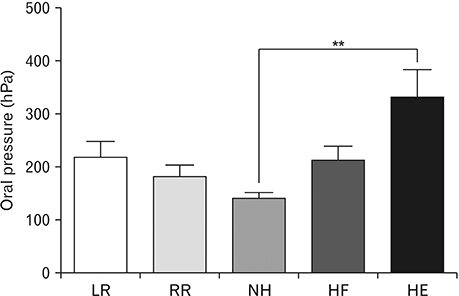
- We present basic data on head positions that can serve as compensatory interventions for patients with weak tongue and buccinator muscles. We studied 30 Korean adults (15 males, 15 females; mean age, 23 years; range, 20-30 years). A TPS-100 instrument was used to measure tongue and cheek pressures and suprahyoid and buccinator muscle activities at various head rotations and tilts, as independent variables. The data were subjected to one-way analysis of variance and post-hoc (linear contrast) testing. Tongue elevation pressures differed significantly when the head was flexed or extended compared to the neutral position (P<0.01). Suprahyoid muscle activity varied significantly when the head was rotated left or right compared to neutral, or tilted with the tongue elevated (P<0.01). Cheek pressure varied significantly when the head was rotated left or right compared to neutral, or tilted (P<0.01). Both tongue and cheek pressures increased significantly when the head was extended or rotated contralaterally compared to the neutral position. Suprahyoid muscle activity increased when the head was flexed or extended, or contralaterally or ipsilaterally rotated compared to the neutral position. Therefore, we suggest that head rotation or tilting could be used to vary oral pressure and muscle activity.
Figure
Cited by 1 articles
-
Association between masticatory muscle activity and oral conditions in young female college students
Cha-Young Pyo, Tae-Hoon Kim, Da-Hye Kim
Anat Cell Biol. 2021;54(4):479-488. doi: 10.5115/acb.21.107.
Reference
-
1. Fregosi RF, Ludlow CL. Activation of upper airway muscles during breathing and swallowing. J Appl Physiol (1985). 2014; 116:291–301.2. Youmans SR, Youmans GL, Stierwalt JA. Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia. 2009; 24:57–65.3. Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: three case reports. Clin Interv Aging. 2008; 3:735–747.4. Moon JH, Kim HJ, Kang MK, Won YS. Effects of tongue strength and accuracy training on tongue strength, swallowing function, and quality of life in chronic stroke patients with dysphagia. J Korea Contents Assoc. 2016; 16:605–613.5. Drake RL, Vogl AW, Mitchell AW. Gray's anatomy for students. 4th ed. Philadelphia, PA: Elsevier/Churchill Livingstone;2019.6. Pendleton HM, Schultz-Krohn W. Pedretti's occupational therapy: practice skills for physical dysfunction. 8th ed. St. Louis, MO.: Mosby/Elsevier;2018.7. Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil. 1989; 70:767–771.8. Lee H, Rho H, Cheon HJ, Oh SM, Kim YH, Chang WH. Selection of head turn side on pharyngeal dysphagia in hemiplegic stroke patients: a preliminary study. Brain Neurorehabil. 2018; 11:e19.9. Kumai Y, Miyamoto T, Matsubara K, Samejima Y, Yoshida N, Baba H, Orita Y. Determining the efficacy of the chin-down maneuver following esophagectomy with fiberoptic endoscopic evaluation of swallowing. Arch Phys Med Rehabil. 2019; 100:1076–1084.10. Criswell E. Cram's introduction to surface electromyography. 2nd ed. Sudbury, MA: Jones and Bartlett Publishers;2011.11. Taniguchi H, Tsukada T, Ootaki S, Yamada Y, Inoue M. Correspondence between food consistency and suprahyoid muscle activity, tongue pressure, and bolus transit times during the oropharyngeal phase of swallowing. J Appl Physiol (1985). 2008; 105:791–799.12. Logemann JA, Curro FA, Pauloski B, Gensler G. Aging effects on oropharyngeal swallow and the role of dental care in oropharyngeal dysphagia. Oral Dis. 2013; 19:733–737.13. Gonzalez-Fernandez M, Ottenstein L, Atanelov L, Christian AB. Dysphagia after stroke: an overview. Curr Phys Med Rehabil Rep. 2013; 1:187–196.14. Huckabee ML, Cannito MP. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: A retrospective evaluation. Dysphagia. 1999; 14:93–109.15. Langenbach GE, Hannam AG. The role of passive muscle tensions in a three-dimensional dynamic model of the human jaw. Arch Oral Biol. 1999; 44:557–573.16. Burbidge AS, Cichero JA, Engmann J, Steele CM. “A day in the life of the fluid bolus”: an introduction to fluid mechanics of the oropharyngeal phase of swallowing with particular focus on dysphagia. Appl Rheol. 2016; 26:64525.17. Walton J, Silva P. Physiology of swallowing. Surgery. 2018; 36:529–534.18. Okeson JP. Management of temporomandibular disorders and occlusion: e-book. 7th ed. St. Louis, MO: Elsevier Health Sciences;2014.19. Cohen EE, LaMonte SJ, Erb NL, Beckman KL, Sadeghi N, Hutcheson KA, Stubblefield MD, Abbott DM, Fisher PS, Stein KD, Lyman GH, Pratt-Chapman ML. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016; 66:203–239.20. Ertekin C, Keskin A, Kiylioglu N, Kirazli Y, On AY, Tarlaci S, Aydogdu I. The effect of head and neck positions on oropharyngeal swallowing: a clinical and electrophysiologic study. Arch Phys Med Rehabil. 2001; 82:1255–1260.21. Schimmel M, Leemann B, Christou P, Kiliaridis S, Herrmann FR, Muller F. Quantitative assessment of facial muscle impairment in patients with hemispheric stroke. J Oral Rehabil. 2011; 38:800–809.22. Logemann JA. Treatment of oral and pharyngeal dysphagia. Phys Med Rehabil Clin N Am. 2008; 19:803–816.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Head-Neck Rotation on Elbow Flexor and Extensor Muscle Activity and Strength in Normal Adults
- An Efficacy of Head Tilt Test in the Patients with Unilateral Superior Oblique Palsy
- Effects of Sling and Resistance Rotation Exercises on Pelvic Rotation and Pain in Patients with Chronic Low Back Pain
- A Case of Congenital Absence of the Superior Oblique Muscle Treated With Anterior and Nasal Transposition of the Inferior Oblique Muscle
- Effects of Head Rotation and Head Tilt on Pharyngeal Pressure Events Using High Resolution Manometry