J Periodontal Implant Sci.
2018 Aug;48(4):202-212. 10.5051/jpis.2018.48.4.202.
Spiral scanning imaging and quantitative calculation of the 3-dimensional screw-shaped bone-implant interface on micro-computed tomography
- Affiliations
-
- 1Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea.
- 2Department of Field Refurbishment, Optoscan, Seoul, Korea.
- 3Department of Prosthodontics, Seoul National University School of Dentistry, Seoul, Korea. pros53@snu.ac.kr
- KMID: 2465386
- DOI: http://doi.org/10.5051/jpis.2018.48.4.202
Abstract
- PURPOSE
Bone-to-implant contact (BIC) is difficult to measure on micro-computed tomography (CT) because of artifacts that hinder accurate differentiation of the bone and implant. This study presents an advanced algorithm for measuring BIC in micro-CT acquisitions using a spiral scanning technique, with improved differentiation of bone and implant materials.
METHODS
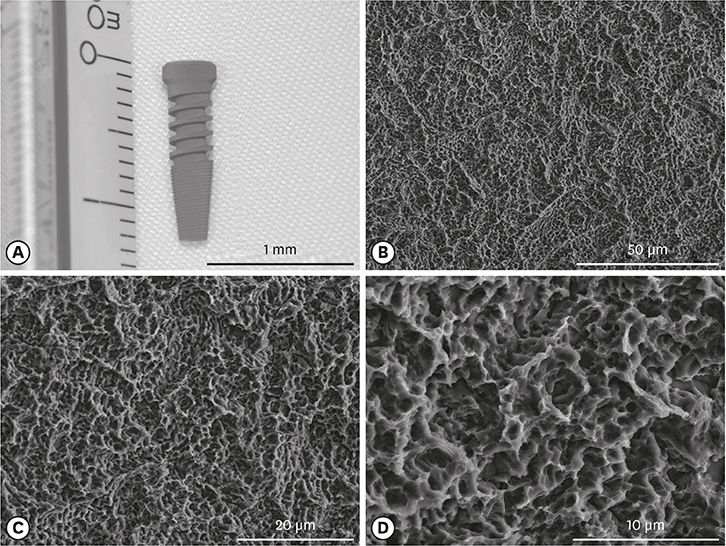
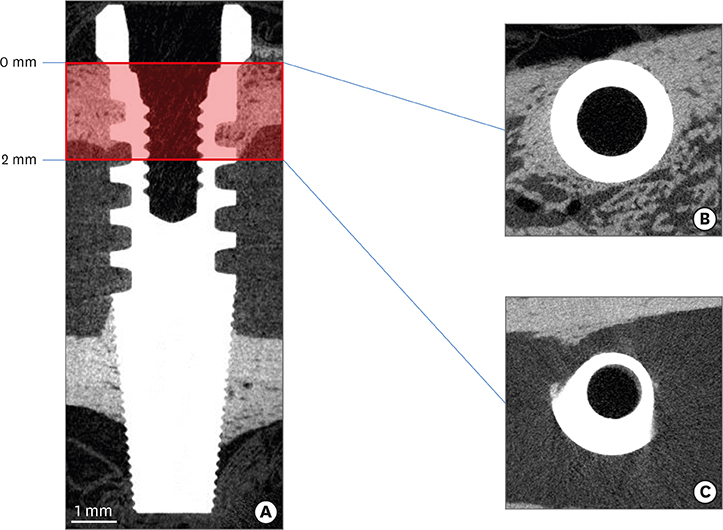
Five sandblasted, large-grit, acid-etched implants were used. Three implants were subjected to surface analysis, and 2 were inserted into a New Zealand white rabbit, with each tibia receiving 1 implant. The rabbit was sacrificed after 28 days. The en bloc specimens were subjected to spiral (SkyScan 1275, Bruker) and round (SkyScan 1172, SkyScan 1275) micro-CT scanning to evaluate differences in the images resulting from the different scanning techniques. The partial volume effect (PVE) was optimized as much as possible. BIC was measured with both round and spiral scanning on the SkyScan 1275, and the results were compared.
RESULTS
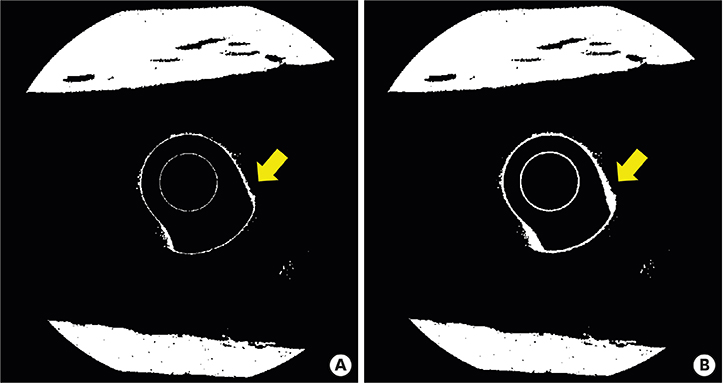
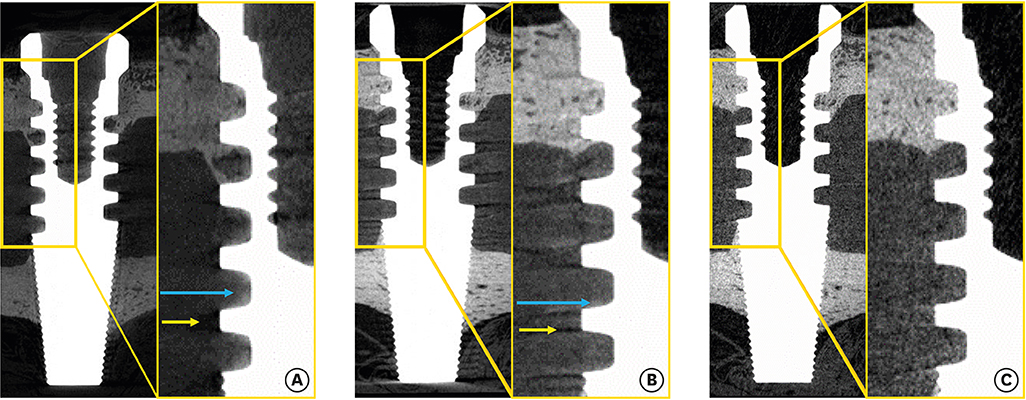
Compared with the round micro-CT scanning, the spiral scanning showed much clearer images. In addition, the PVE was optimized, which allowed accurate BIC measurements to be made. Round scanning on the SkyScan 1275 resulted in higher BIC measurements than spiral scanning on the same machine; however, the higher measurements on round scanning were confirmed to be false, and were found to be the result of artifacts in the void, rather than bone.
CONCLUSIONS
The results of this study indicate that spiral scanning can reduce metal artifacts, thereby allowing clear differentiation of bone and implant. Moreover, the PVE, which is a factor that inevitably hinders accurate BIC measurements, was optimized through an advanced algorithm.
Keyword
Figure
Cited by 1 articles
-
How do imaging protocols affect the assessment of root-end fillings?
Fernanda Ferrari Esteves Torres, Reinhilde Jacobs, Mostafa EzEldeen, Karla de Faria-Vasconcelos, Juliane Maria Guerreiro-Tanomaru, Bernardo Camargo dos Santos, Mário Tanomaru-Filho
Restor Dent Endod. 2021;47(1):e2. doi: 10.5395/rde.2022.47.e2.
Reference
-
1. Bernhardt R, Kuhlisch E, Schulz MC, Eckelt U, Stadlinger B. Comparison of bone-implant contact and bone-implant volume between 2D-histological sections and 3D-SRµCT slices. Eur Cell Mater. 2012; 23:237–247.
Article2. Bissinger O, Probst FA, Wolff KD, Jeschke A, Weitz J, Deppe H, et al. Comparative 3D micro-CT and 2D histomorphometry analysis of dental implant osseointegration in the maxilla of minipigs. J Clin Periodontol. 2017; 44:418–427.
Article3. Jimbo R, Coelho PG, Vandeweghe S, Schwartz-Filho HO, Hayashi M, Ono D, et al. Histological and three-dimensional evaluation of osseointegration to nanostructured calcium phosphate-coated implants. Acta Biomater. 2011; 7:4229–4234.
Article4. Vandeweghe S, Coelho PG, Vanhove C, Wennerberg A, Jimbo R. Utilizing micro-computed tomography to evaluate bone structure surrounding dental implants: a comparison with histomorphometry. J Biomed Mater Res B Appl Biomater. 2013; 101:1259–1266.
Article5. Becker K, Stauber M, Schwarz F, Beißbarth T. Automated 3D-2D registration of X-ray microcomputed tomography with histological sections for dental implants in bone using chamfer matching and simulated annealing. Comput Med Imaging Graph. 2015; 44:62–68.
Article6. Bernhardt R, Scharnweber D, Müller B, Thurner P, Schliephake H, Wyss P, et al. Comparison of microfocus- and synchrotron X-ray tomography for the analysis of osteointegration around Ti6Al4V implants. Eur Cell Mater. 2004; 7:42–51.
Article7. Park YS, Yi KY, Lee IS, Jung YC. Correlation between microtomography and histomorphometry for assessment of implant osseointegration. Clin Oral Implants Res. 2005; 16:156–160.
Article8. Barrett JF, Keat N. Artifacts in CT: recognition and avoidance. Radiographics. 2004; 24:1679–1691.
Article9. Li JY, Pow EH, Zheng LW, Ma L, Kwong DL, Cheung LK. Quantitative analysis of titanium-induced artifacts and correlated factors during micro-CT scanning. Clin Oral Implants Res. 2014; 25:506–510.
Article10. Narra N, Antalainen AK, Zipprich H, Sándor GK, Wolff J. Microcomputed tomography-based assessment of retrieved dental implants. Int J Oral Maxillofac Implants. 2015; 30:308–314.
Article11. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage. 2012; 20:256–260.
Article12. Salmon P. Micro-CT image analysis techniques for orthopedic applications: metal implant-to-bone contact surface and porosity of biomaterials. In : Leung KS, Qin L, Cheung WH, editors. A practical manual for musculoskeletal research. Singapore: World Scientific;2008. p. 583–603.13. Rebaudi A, Koller B, Laib A, Trisi P. Microcomputed tomographic analysis of the peri-implant bone. Int J Periodontics Restorative Dent. 2004; 24:316–325.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Torque and mechanical failure of orthodontic micro-implant influenced by implant design parameters
- Three-dimensional imaging modalities in endodontics
- Micro-computed tomography for assessing the internal and external voids of bulk-fill composite restorations: A technical report
- THREE DIMENSIONAL FINITE ELEMENT ANALYSIS ON THE MINMUM CONTACT FRACTION OF BONE-IMPLANT INTERFACE
- Bone height measurements of implant sites: Comparison of panoramic radiography and spiral computed tomography