Korean J Ophthalmol.
2019 Dec;33(6):493-499. 10.3341/kjo.2019.0063.
Effect of Acetazolamide on Choroidal Morphology in Central Serous Chorioretinopathy
- Affiliations
-
- 1Department of Ophthalmology, Bucheon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Bucheon, Korea. 20900102@cmcnu.ac.kr
- KMID: 2465128
- DOI: http://doi.org/10.3341/kjo.2019.0063
Abstract
- PURPOSE
We sought to elucidate the influence of acetazolamide on choroidal structure changes during the treatment of central serous chorioretinopathy (CSC).
METHODS
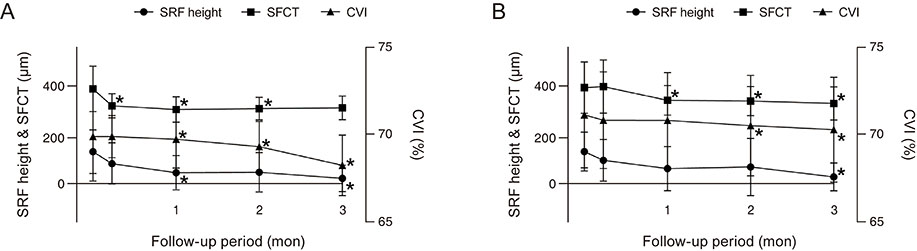
This was a retrospective study of 45 eyes from 45 patients with acute CSC who were divided into an acetazolamide group (group 1, n = 20) and an observation group (group 2, n = 25). The main outcome measures were the changes in best-corrected visual acuity, subretinal fluid (SRF) height, subfoveal choroidal thickness (SFCT), and choroidal vascularity index (CVI) at one week, one month, two months, and three months, respectively.
RESULTS
Although statistical significance was not reached, best-corrected visual acuity improved in both groups at month 3 (from 0.06 ± 0.07 to 0.01 ± 0.03 in group 1 and 0.17 ± 0.24 to 0.09 ± 0.18 in group 2; p = 0.083 and 0.183, respectively). Separately, SRF height and CVI showed a significant decrease at three months in both groups (all p < 0.05), while a significant SRF height decrease was also noted in group 1 at one month (p = 0.038). In group 1, a significant decrease in the SFCT and CVI started at one week and one month (p = 0.021 and 0.008), respectively. However, in group 2, a significant decrease in the SFCT and CVI started at one month and two months (p = 0.005 and 0.015), respectively.
CONCLUSIONS
Acetazolamide has no effect on final functional or anatomical status at three months in eyes with CSC but does shorten the time for SRF absorption and accompanying choroidal structural changes.
Keyword
MeSH Terms
Figure
Reference
-
1. Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol. 2008; 86:126–145.2. Manayath GJ, Ranjan R, Karandikar SS, et al. Central serous chorioretinopathy: current update on management. Oman J Ophthalmol. 2018; 11:200–206.3. Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988; 106:1190–1195.4. Pikkel J, Beiran I, Ophir A, Miller B. Acetazolamide for central serous retinopathy. Ophthalmology. 2002; 109:1723–1725.5. Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009; 29:1469–1473.6. Tan KA, Agrawal R. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 2015; 160:394.7. Agrawal R, Chhablani J, Tan KA, et al. Choroidal vascularity index in central serous chorioretinopathy. Retina. 2016; 36:1646–1651.8. Sonoda S, Sakamoto T, Yamashita T, et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. 2014; 55:3893–3899.9. Agrawal R, Gupta P, Tan KA, et al. Choroidal vascularity index as a measure of vascular status of the choroid: measurements in healthy eyes from a population-based study. Sci Rep. 2016; 6:21090.10. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996; 103:2070–2080.11. Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994; 112:1057–1062.12. Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996; 16:203–213.13. Shiraki K, Moriwaki M, Matsumoto M, et al. Long-term follow-up of severe central serous chorioretinopathy using indocyanine green angiography. Int Ophthalmol. 1997-1998; 21:245–253.14. Iida T, Kishi S, Hagimura N, Shimizu K. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999; 19:508–512.15. Spaide RF, Goldbaum M, Wong DW, et al. Serous detachment of the retina. Retina. 2003; 23:820–846.16. Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996; 121:26–34.17. Prunte C. Indocyanine green angiographic findings in central serous chorioretinopathy. Int Ophthalmol. 1995; 19:77–82.18. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146:496–500.19. Brandl C, Helbig H, Gamulescu MA. Choroidal thickness measurements during central serous chorioretinopathy treatment. Int Ophthalmol. 2014; 34:7–13.20. Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol. 1999; 97:387–397.21. Wolfensberger TJ, Dmitriev AV, Govardovskii VI. Inhibition of membrane-bound carbonic anhydrase decreases subretinal pH and volume. Doc Ophthalmol. 1999; 97:261–271.22. Wolfensberger TJ, Chiang RK, Takeuchi A, Marmor MF. Inhibition of membrane-bound carbonic anhydrase enhances subretinal fluid absorption and retinal adhesiveness. Graefes Arch Clin Exp Ophthalmol. 2000; 238:76–80.23. Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011; 31:1603–1608.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laser-Induced Choroidal Neovascularization in Central Serous Chorioretinopathy: 4 Cases
- A Case of Focal Choroidal Excavation Associated with Chronic Central Serous Chorioretinopathy
- Electronmicroscopic Study of the Effect of Hexamethonium on Serous Choriretinopathy in Rabbits
- Indocyanine Green Angiographics Findings in Central Serous Chorioretinopathy
- Chroidal Circulation in Central Serous Chorioretinopathy using Indocyanine Green Angiography