J Korean Neurosurg Soc.
2019 Nov;62(6):635-642. 10.3340/jkns.2019.0122.
Comparison of Biomechanical Properties of Dura Mater Substitutes and Cranial Human Dura Mater : An In Vitro Study
- Affiliations
-
- 1Department of Neurosurgery, Dokuz Eylul University School of Medicine, Izmir, Turkey. ceren.kizmazoglu@gmail.com
- 2Department of Neurosurgery, Kutahya Health Science University Evliya Celebi Training and Research Hospital, Kutahya, Turkey.
- 3Department of Neurosurgery, Sultan Abdulhamid Han Training and Research Hospital, Istanbul, Turkey.
- 4Department of Biomechanics, Dokuz Eylul University School of Medicine Health Science Institute, Izmir, Turkey.
- 5Department of Pathology, Forensic Medicine Institution, Izmir, Turkey.
- 6Department of Orthopedics and Traumatology, Dokuz Eylul University School of Medicine, Izmir, Turkey.
- KMID: 2463655
- DOI: http://doi.org/10.3340/jkns.2019.0122
Abstract
OBJECTIVE
The aim of this study was to investigate the biomechanical differences between human dura mater and dura mater substitutes to optimize biomimetic materials.
METHODS
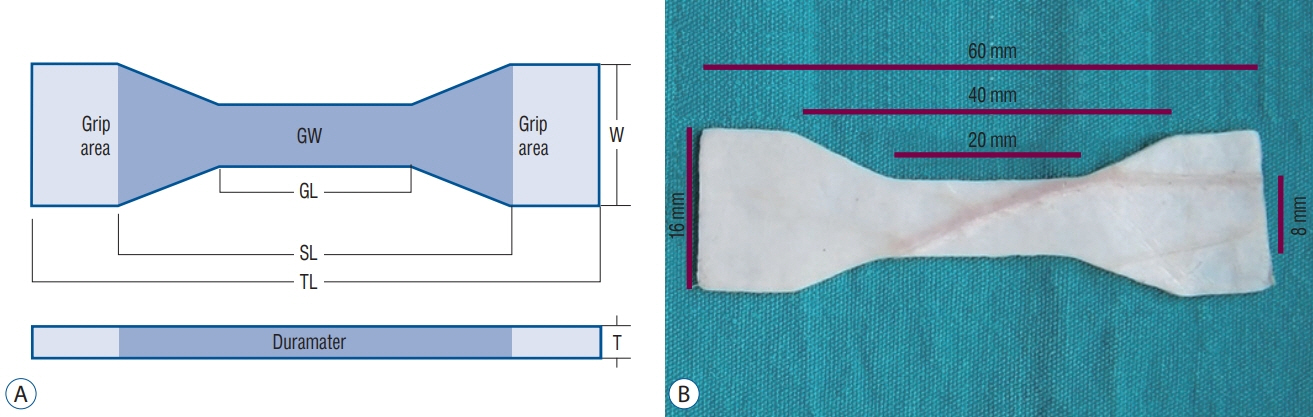
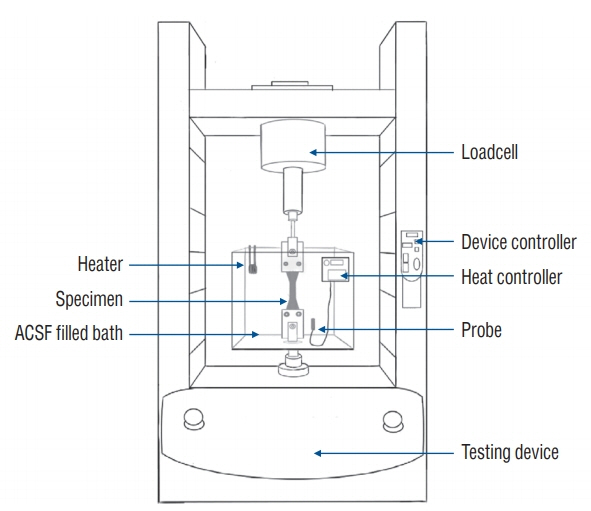
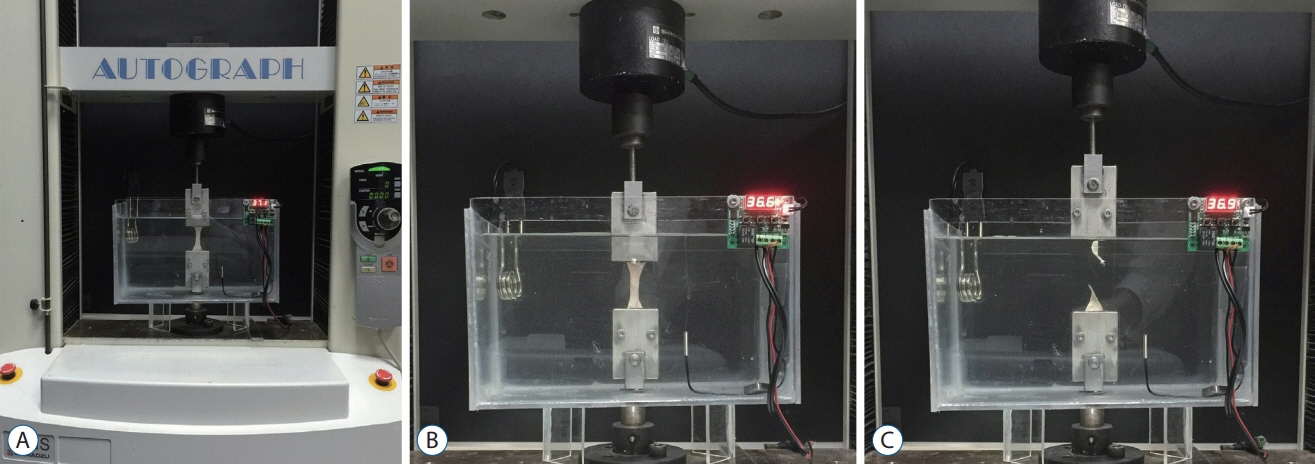
Four groups were investigated. Group I used cranial dura mater (n=10), group II used Gore-Tex® Expanded Cardiovascular Patch (W.L. Gore & Associates Inc., Flagstaff, AZ, USA) (n=6), group III used Durepair® (Medtronic Inc., Goleta, CA, USA) (n=6), and group IV used Tutopatch® (Tutogen Medical GmbH, Neunkirchen am Brand, Germany) (n=6). We used an axial compression machine to measure maximum tensile strength.
RESULTS
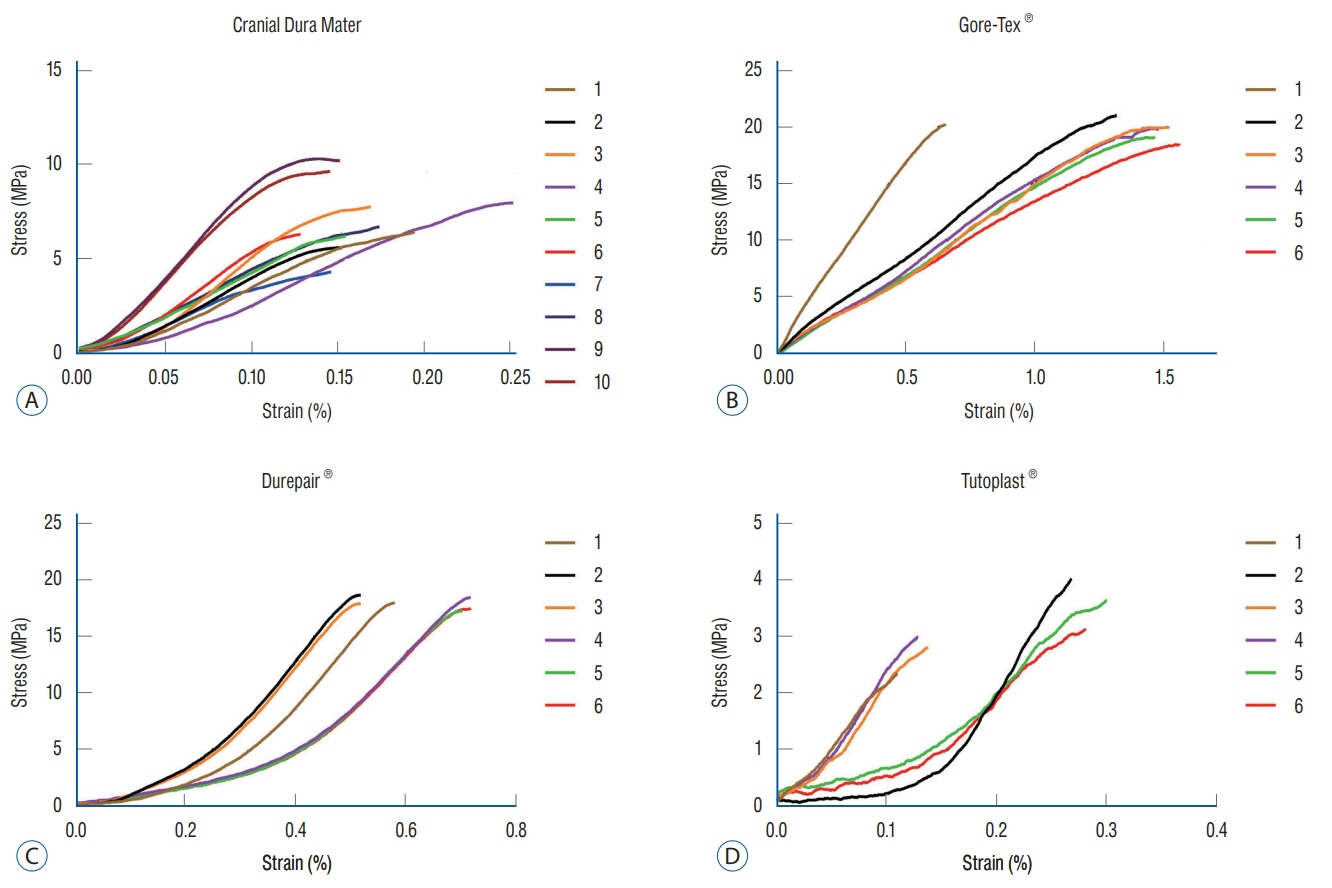
The mean tensile strengths were 7.01±0.77 MPa for group I, 22.03±0.60 MPa for group II, 19.59±0.65 MPa for group III, and 3.51±0.63 MPa for group IV. The materials in groups II and III were stronger than those in group I. However, the materials in group IV were weaker than those in group I.
CONCLUSION
An important dura mater graft property is biomechanical similarity to cranial human dura mater. This biomechanical study contributed to the future development of artificial dura mater substitutes with biomechanical properties similar to those of human dura mater.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Aliredjo RP, de Vries J, Menovsky T, Grotenhuis JA, Merx J. The use of Gore-Tex membrane for adhesion prevention in tethered spinal cord surgery: technical case reports. Neurosurgery. 44:674–677. 1999.
Article2. Azzam D, Romiyo P, Nguyen T, Sheppard JP, Alkhalid Y, Lagman C, et al. Dural repair in cranial surgery is associated with moderate rates of complications with both autologous and nonautologous dural substitutes. World Neurosurg. 113:244–248. 2018.
Article3. Cantore G, Guidetti B, Delfini R. Neurosurgical use of human dura mater sterilized by gamma rays and stored in alcohol: long-term results. J Neurosurg. 66:93–95. 1987.
Article4. Chaplin JM, Costantino PD, Wolpoe ME, Bederson JB, Griffey ES, Zhang WX. Use of an acellular dermal allograft for dural replacement: an experimental study. Neurosurgery. 45:320–327. 1999.
Article5. Danish SF, Samdani A, Hanna A, Storm P, Sutton L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J Neurosurg. 104:16–20. 2006.
Article6. Famaey N, Verhoeven J, Jacobs S, Pettinari M, Meyns B. In situ evolution of the mechanical properties of stretchable and non-stretchable ePTFE vascular grafts and adjacent native vessels. Int J Artif Organs. 37:900–910. 2014.
Article7. Filippi R, Schwarz M, Voth D, Reisch R, Grunert P, Perneczky A. Bovine pericardium for duraplasty: clinical results in 32 patients. Neurosurg Rev. 24:103–107. 2001.
Article8. Fung YC. Biomechanics: mechanical properties of living tissues. ed 2. New York: Springer-Verlag;1981.9. Hoshi K, Yoshino H, Urata J, Nakamura Y, Yanagawa H, Sato T. Creutzfeldt-Jakob disease associated with cadaveric dura mater grafts in Japan. Neurology. 55:718–721. 2000.
Article10. Inoue HK, Kobayashi S, Ohbayashi K, Kohga H, Nakamura M. Treatment and prevention of tethered and retethered spinal cord using a Gore-Tex surgical membrane. J Neurosurg. 80:689–693. 1994.
Article11. Knopp U, Christmann F, Reusche E, Sepehrnia A. A new collagen biomatrix of equine origin versus a cadaveric dura graft for the repair of dural defects--a comparative animal experimental study. Acta Neurochir (Wien). 147:877–887. 2005.
Article12. Laun A, Tonn JC, Jerusalem C. Comparative study of lyophilized human dura mater and lyophilized bovine pericardium as dural substitutes in neurosurgery. Acta Neurochir (Wien). 107:16–21. 1990.
Article13. Lee CK, Alexander H. Prevention of postlaminectomy scar formation. Spine (Phila Pa 1976). 9:305–312. 1984.
Article14. McGarvey KA, Lee JM, Boughner DR. Mechanical suitability of glycerolpreserved human dura mater for construction of prosthetic cardiac valves. Biomaterials. 5:109–117. 1984.
Article15. Moskowitz SI, Liu J, Krishnaney AA. Postoperative complications associated with dural substitutes in suboccipital craniotomies. Neurosurgery. 64:28–33. 2009.
Article16. Patin DJ, Eckstein EC, Harum K, Pallares VS. Anatomic and biomechanical properties of human lumbar dura mater. Anesth Analg. 76:535–540. 1993.
Article17. Protasoni M, Sangiorgi S, Cividini A, Culuvaris GT, Tomei G, Dell’Orbo C, et al. The collagenic architecture of human dura mater. J Neurosurg. 114:1723–1730. 2011.
Article18. Runza M, Pietrabissa R, Mantero S, Albani A, Quaglini V, Contro R. Lumbar dura mater biomechanics: experimental characterization and scanning electron microscopy observations. Anesth Analg. 88:1317–1321. 1999.19. Sacks MS, Jimenez Hamann MC, Otaño-Lata SE, Malinin TI. Local mechanical anisotropy in human cranial dura mater allografts. J Biomech Eng. 120:541–544. 1998.
Article20. Sade B, Oya S, Lee JH. Non-watertight dural reconstruction in meningioma surgery: results in 439 consecutive patients and a review of the literature. Clinical article. J Neurosurg. 114:714–718. 2011.
Article21. Topsakal C, Akpolat N, Erol FS, Ozveren MF, Akdemir I, Kaplan M, et al. Seprafilm superior to Gore-Tex in the prevention of peridural fibrosis. J Neurosurg. 101:295–302. 2004.
Article22. van Noort R, Black MM, Martin TR, Meanley S. A study of the uniaxial mechanical properties of human dura mater preserved in glycerol. Biomaterials. 2:41–45. 1981.
Article23. Warren WL, Medary MB, Dureza CD, Bellotte JB, Flannagan PP, Oh MY, et al. Dural repair using acellular human dermis: experience with 200 cases: technique assessment. Neurosurgery. 46:1391–1396. 2000.
Article24. Wilcox RK, Bilston LE, Barton DC, Hall RM. Mathematical model for the viscoelastic properties of dura mater. J Orthop Sci. 8:432–434. 2003.
Article25. Wolfinbarger L, Zhang YX, Adam BLT, Homsi D, Gates K, Sutherland V. Biomechanical aspects on rehydrated freeze-dried human allograft dura mater tissues. J Appl Biomater. 5:265–270. 1994.
Article26. Yamada K, Miyamoto S, Nagata I, Kikuchi H, Ikada Y, Iwata H, et al. Development of a dural substitute from synthetic bioabsorbable polymers. J Neurosurg. 86:1012–1017. 1997.
Article27. Yamada K, Miyamoto S, Takayama M, Nagata I, Hashimoto N, Ikada Y, et al. Clinical application of a new bioabsorbable artificial dura mater. J Neurosurg. 96:731–735. 2002.
Article28. Zerris VA, James KS, Roberts JB, Bell E, Heilman CB. Repair of the dura mater with processed collagen devices. J Biomed Mater Res B Appl Biomater. 83:580–588. 2007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biomechanical Properties of the Cranial Dura Mater with Puncture Defects: An In Vitro Study
- In vitro Fusion of the Posterior Frontal Calvarial Suture in the Mouse
- Lyophilized Dura Mater Patch Graft in Glaucoma Valve Implantation
- Idiopathic Hypertrophic Cranial Pachymeningitis: Case Report
- Idiopathic Hypertrophic Pachymeningitis in the Craniocervical Junction