Ann Surg Treat Res.
2019 Nov;97(5):245-253. 10.4174/astr.2019.97.5.245.
Hepatic resection after neoadjuvant chemotherapy for patients with liver metastases from colorectal cancer: need for cautious planning
- Affiliations
-
- 1Department of Colon and Rectal Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ipark@amc.seoul.kr
- 2Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Division of Hepatobiliary and Pancreatic Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 6Division of Hepatobiliary Surgery and Liver Transplantation, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2461900
- DOI: http://doi.org/10.4174/astr.2019.97.5.245
Abstract
- PURPOSE
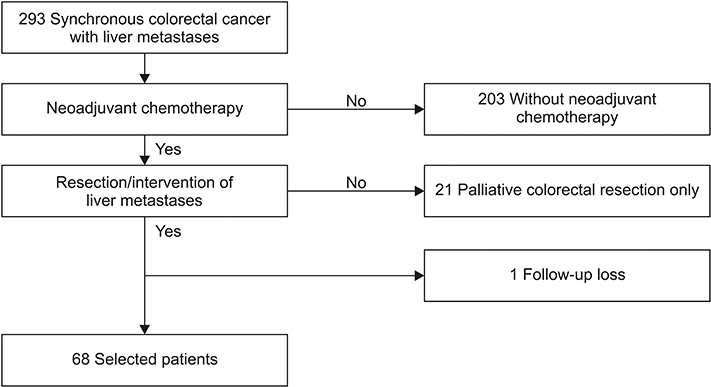
Current neoadjuvant chemotherapy (NAC) may enable therapies such as surgical resection and local ablation of metastases in patients with colorectal liver metastasis (CLM). We evaluated outcomes in CLM patients who underwent resection and/or local treatment after NAC and identified prognostic factors for oncologic outcomes.
METHODS
Patients who received NAC followed by resection and/or local treatment of hepatic metastasis from 2013 to 2015 were included. Treatment and tumor-related variables were tabulated. Recurrence-free survival (RFS) and overall survival (OS) were analyzed using the Kaplan-Meier method. Cox regression analysis was used to identify factors associated with RFS and OS.
RESULTS
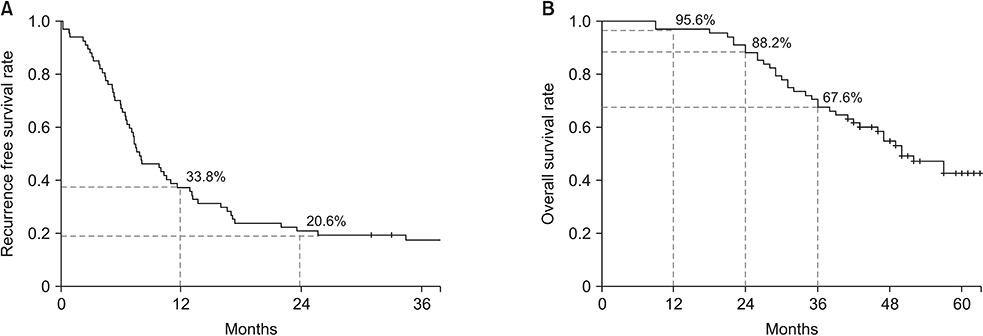
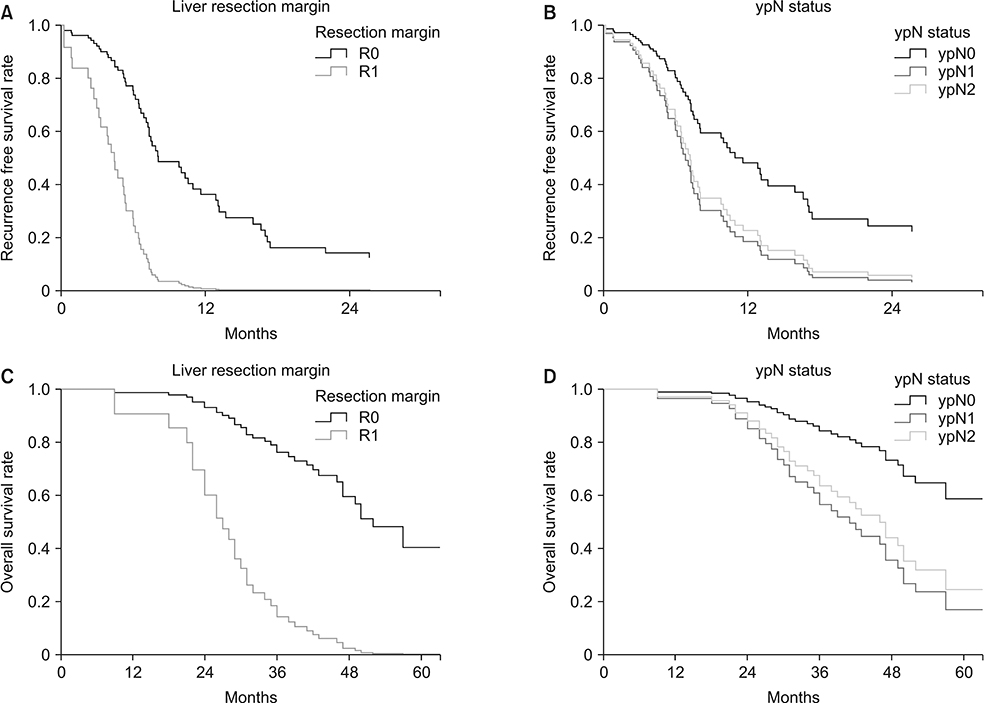
Sixty-eight patients received NAC followed by resection and/or local treatment of hepatic metastases. Targeted therapy was administered in 50% of the patients. RFS was 35.8% at 1 year and 19.4% at 2 years postoperatively. OS was 95.6% at 1 year and 88.2% at 2 years postoperatively. In the multivariable analysis, R1 resection margin (hazard ratio [HR], 3.95; P = 0.008) of the liver metastases and ypN1/ypN2 (HR, 2.356 and 1.983, respectively; P = 0.041) were associated with poor RFS. Both factors were also significantly related to OS.
CONCLUSION
Resection margin of the metastatic tumor and ypN status are the only relevant factors for RFS and OS in CLM patients treated with NAC. Despite early and high rates of recurrence, CLM patients treated with NAC who undergo resection and/or local treatment have acceptable OS. Multidisciplinary review of candidates for surgery and cautious planning are crucial for achieving optimal outcomes.
Figure
Cited by 1 articles
-
Adjuvant oxaliplatin-based chemotherapy effect after treatment of colorectal hepatic metastasis
Mee-Young Kang, Jin-Hee Paik, Chun-Geun Ryu, Dae-Yong Hwang
Ann Surg Treat Res. 2021;101(3):160-166. doi: 10.4174/astr.2021.101.3.160.
Reference
-
1. Bengtsson G, Carlsson G, Hafstrom L, Jonsson PE. Natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg. 1981; 141:586–589.
Article2. Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996; 77:1254–1262.3. Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996; 224:509–520.
Article4. Hallet J, Sa Cunha A, Adam R, Goere D, Bachellier P, Azoulay D, et al. Factors influencing recurrence following initial hepatectomy for colorectal liver metastases. Br J Surg. 2016; 103:1366–1376.5. Allen PJ, Kemeny N, Jarnagin W, DeMatteo R, Blumgart L, Fong Y. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003; 7:109–117.
Article6. Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004; 240:1052–1061.7. Gomez D, Cameron IC. Prognostic scores for colorectal liver metastasis: clinically important or an academic exercise? HPB (Oxford). 2010; 12:227–238.
Article8. Gallagher DJ, Zheng J, Capanu M, Haviland D, Paty P, Dematteo RP, et al. Response to neoadjuvant chemotherapy does not predict overall survival for patients with synchronous colorectal hepatic metastases. Ann Surg Oncol. 2009; 16:1844–1851.
Article9. de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008; 248:626–637.10. Poultsides GA, Schulick RD, Pawlik TM. Hepatic resection for colorectal metastases: the impact of surgical margin status on outcome. HPB (Oxford). 2010; 12:43–49.
Article11. Vigano L, Procopio F, Cimino MM, Donadon M, Gatti A, Costa G, et al. Is tumor detachment from vascular structures equivalent to R0 resection in surgery for colorectal liver metastases? An observational cohort. Ann Surg Oncol. 2016; 23:1352–1360.
Article12. Margonis GA, Buettner S, Andreatos N, Sasaki K, Ijzermans JNM, van Vugt JLA, et al. Anatomical resections improve disease-free survival in patients with KRAS-mutated colorectal liver metastases. Ann Surg. 2017; 266:641–649.
Article13. Cucchetti A, Russolillo N, Johnson P, Tarchi P, Ferrero A, Cucchi M, et al. Impact of primary cancer features on behaviour of colorectal liver metastases and survival after hepatectomy. BJS Open. 2018; 3:186–194.
Article14. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.
Article15. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999; 230:309–318.16. Nagashima I, Takada T, Adachi M, Nagawa H, Muto T, Okinaga K. Proposal of criteria to select candidates with colorectal liver metastases for hepatic resection: comparison of our scoring system to the positive number of risk factors. World J Gastroenterol. 2006; 12:6305–6309.
Article17. Konopke R, Kersting S, Distler M, Dietrich J, Gastmeier J, Heller A, et al. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009; 29:89–102.
Article18. Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B, et al. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008; 143:384–393.
Article19. Jung SW, Kim DS, Yu YD, Han JH, Suh SO. Risk factors for cancer recurrence or death within 6 months after liver resection in patients with colorectal cancer liver metastasis. Ann Surg Treat Res. 2016; 90:257–264.
Article20. Wakai T, Shirai Y, Sakata J, Valera VA, Korita PV, Akazawa K, et al. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol. 2008; 15:2472–2481.21. Wray CJ, Lowy AM, Mathews JB, Park S, Choe KA, Hanto DW, et al. The significance and clinical factors associated with a subcentimeter resection of colorectal liver metastases. Ann Surg Oncol. 2005; 12:374–380.
Article22. Cady B, Jenkins RL, Steele GD Jr, Lewis WD, Stone MD, McDermott WV, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998; 227:566–571.23. Figueras J, Burdio F, Ramos E, Torras J, Llado L, Lopez-Ben S, et al. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol. 2007; 18:1190–1195.
Article24. Ayez N, Lalmahomed ZS, Eggermont AM, Ijzermans JN, de Jonge J, van Montfort K, et al. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol. 2012; 19:1618–1627.
Article25. Weiser MR, Jarnagin WR, Saltz LB. Colorectal cancer patients with oligometastatic liver disease: what is the optimal approach? Oncology (Williston Park). 2013; 27:1074–1078.26. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016; 27:1386–1422.
Article27. Serayssol C, Maulat C, Breibach F, Mokrane FZ, Selves J, Guimbaud R, et al. Predictive factors of histological response of colorectal liver metastases after neoadjuvant chemotherapy. World J Gastrointest Oncol. 2019; 11:295–309.
Article28. Wimmer K, Schwarz C, Szabo C, Bodingbauer M, Tamandl D, Mittlbock M, et al. Impact of neoadjuvant chemotherapy on clinical risk scores and survival in patients with colorectal liver metastases. Ann Surg Oncol. 2017; 24:236–243.
Article29. Ayez N, Lalmahomed ZS, van der Pool AE, Vergouwe Y, van Montfort K, de Jonge J, et al. Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy? Ann Surg Oncol. 2011; 18:2757–2763.
Article30. Wein A, Riedel C, Bruckl W, Merkel S, Ott R, Hanke B, et al. Neoadjuvant treatment with weekly high-dose 5-Fluorouracil as 24-hour infusion, folinic acid and oxaliplatin in patients with primary resectable liver metastases of colorectal cancer. Oncology. 2003; 64:131–138.
Article