J Korean Assoc Oral Maxillofac Surg.
2019 Feb;45(1):34-42. 10.5125/jkaoms.2019.45.1.34.
Natural bioceramics: our experience with changing perspectives in the reconstruction of maxillofacial skeleton
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Sibar Institute of Dental Sciences, Guntur, India. drvivekanandsk@gmail.com
- KMID: 2461490
- DOI: http://doi.org/10.5125/jkaoms.2019.45.1.34
Abstract
OBJECTIVES
Various bone graft substitute materials are used to enhance bone regeneration in the maxillofacial skeleton. In the recent past, synthetic graft materials have been produced using various synthetic and natural calcium precursors. Very recently, eggshell-derived hydroxyapatite (EHA) has been evaluated as a synthetic bone graft substitute. To assess bone regeneration using EHA in cystic and/or apicectomy defects of the jaws through clinical and radiographic evaluations.
MATERIALS AND METHODS
A total of 20 patients were enrolled in the study protocol (CTRI/2014/12/005340) and were followed up at 4, 8, 12, and 24 weeks to assess the amount of osseous fill through digital radiographs/cone-beam computed tomography along with clinical parameters and complications. Wilcoxon matched pairs test, means, percentages and standard deviations were used for the statistical analysis.
RESULTS
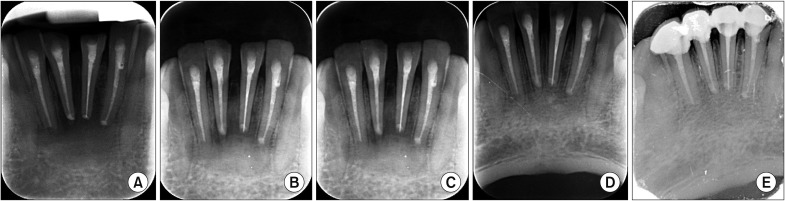
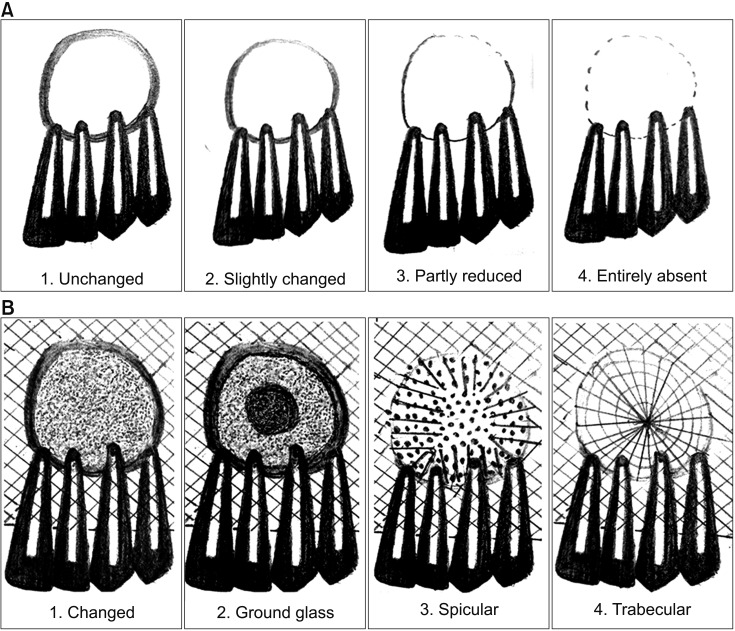
The sizes of the lesions in the study ranged from 1 to 4 cm and involved one to four teeth. The study showed significant changes in the formation of bone, the merging of material and the surgical site margins from the first week to the first month in all patients (age range, 15-50 years) irrespective of the size of the lesions and the number of teeth involved. Bone formation was statistically significant from the fourth to the eighth week, and the trabecular pattern was observed by the end of 12 weeks with uneventful wound healing.
CONCLUSION
EHA showed enhancement of bone regeneration, and healing was complete by the end of 12 weeks with a trabecular pattern in all patients irrespective of the size of the lesion involved. The study showed enhancement of bone regeneration in the early bone formative stage within 12 weeks after grafting. EHA is cost effective and production is environment friendly with no disease transfer risks. Thus, natural bioceramics will play an important role in the reduction of costs involved in grafting and reconstruction.
Keyword
Figure
Cited by 1 articles
-
Socket preservation using eggshell-derived nanohydroxyapatite with platelet-rich fibrin as a barrier membrane: a new technique
Vivekanand Sabanna Kattimani, Krishna Prasad Lingamaneni, Girija Easwaradas Kreedapathi, Kiran Kumar Kattappagari
J Korean Assoc Oral Maxillofac Surg. 2019;45(6):332-342. doi: 10.5125/jkaoms.2019.45.6.332.
Reference
-
1. Thrivikraman G, Athirasala A, Twohig C, Boda SK, Bertassoni LE. Biomaterials for craniofacial bone regeneration. Dent Clin North Am. 2017; 61:835–856. PMID: 28886771.
Article2. Gilbert Triplett R, Budinskaya O. New frontiers in biomaterials. Oral Maxillofac Surg Clin North Am. 2017; 29:105–115. PMID: 27890224.
Article3. Adamopoulos O, Papadopoulos T. Nanostructured bioceramics for maxillofacial applications. J Mater Sci Mater Med. 2007; 18:1587–1597. PMID: 17483893.
Article4. Kattimani VS, Chakravarthi PS, Prasad LK. Biograft block hydroxyapatite: a ray of hope in the reconstruction of maxillofacial defects. J Craniofac Surg. 2016; 27:247–252. PMID: 26745193.5. Kattimani VS, Chakravarthi PS, Kanumuru NR, Subbarao VV, Sidharthan A, Kumar TS, et al. Eggshell derived hydroxyapatite as bone graft substitute in the healing of maxillary cystic bone defects: a preliminary report. J Int Oral Health. 2014; 6:15–19. PMID: 25083027.6. Kattimani V, Lingamaneni KP, Chakravarthi PS, Kumar TS, Siddharthan A. Eggshell-derived hydroxyapatite: a new era in bone regeneration. J Craniofac Surg. 2016; 27:112–117. PMID: 26674907.7. Siva Rama Krishna D, Siddharthan A, Seshadri SK, Sampath Kumar TS. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J Mater Sci Mater Med. 2007; 18:1735–1743. PMID: 17483877.
Article8. Suresh Kumar G, Girija EK. Flower-like hydroxyapatite nanostructure obtained from eggshell: a candidate for biomedical applications. Ceram Int. 2013; 39:8293–8299.9. Kattimani VS, Bajantai NV, Sriram SK, Sriram RR, Rao VK, Desai PD. Observer strategy and radiographic classification of healing after grafting of cystic defects in maxilla: a radiological appraisal. J Contemp Dent Pract. 2013; 14:227–232. PMID: 23811650.10. Kattimani VS, Chakravarthi SP, Neelima Devi KN, Sridhar MS, Prasad LK. Comparative evaluation of bovine derived hydroxyapatite and synthetic hydroxyapatite graft in bone regeneration of human maxillary cystic defects: a clinico-radiological study. Indian J Dent Res. 2014; 25:594–601. PMID: 25511058.
Article11. Dupoirieux L, Pourquier D, Souyris F. Powdered eggshell: a pilot study on a new bone substitute for use in maxillofacial surgery. J Craniomaxillofac Surg. 1995; 23:187–194. PMID: 7673447.
Article12. Baliga M, Davies P, Dupoirieux L. [La poudre de coquille d'oeuf dans le comblement des cavitescystiques des maxillaires]. Rev Stomatol Chir Maxillofac. 1998; 99(Suppl 1):86–88. French. PMID: 9697237.13. Dupoirieux L. Ostrich eggshell as a bone substitute: a preliminary report of its biological behaviour in animals--a possibility in facial reconstructive surgery. Br J Oral Maxillofac Surg. 1999; 37:467–471. PMID: 10687909.14. Dupoirieux L, Neves M, Pourquier D. Comparison of pericranium and eggshell as space fillers used in combination with guided bone regeneration: an experimental study. J Oral Maxillofac Surg. 2000; 58:40–46. discussion 47-8. PMID: 10632164.
Article15. Park JW, Bae SR, Suh JY, Lee DH, Kim SH, Kim H, et al. Evaluation of bone healing with eggshell-derived bone graft substitutes in rat calvaria: a pilot study. J Biomed Mater Res A. 2008; 87:203–214. PMID: 18085653.
Article16. Kim SH, Kim W, Cho JH, Oh NS, Lee MH, Lee SJ. Comparison of bone formation in rabbits using hydroxyapatite and β-tricalcium phosphate scaffolds fabricated from egg shells. Adv Mater Res. 2008; 47-50:999–1002.
Article17. Lee SW, Kim SG, Balázsi C, Chae WS, Lee HO. Comparative study of hydroxyapatite from eggshells and synthetic hydroxyapatite for bone regeneration. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:348–355. PMID: 22676827.
Article18. El-Ghannam A, Amin H, Nasr T, Shama A. Enhancement of bone regeneration and graft material resorption using surface-modified bioactive glass in cortical and human maxillary cystic bone defects. Int J Oral Maxillofac Implants. 2004; 19:184–191. PMID: 15101588.19. Matsuoka H, Akiyama H, Okada Y, Ito H, Shigeno C, Konishi J, et al. In vitro analysis of the stimulation of bone formation by highly bioactive apatite- and wollastonite-containing glass-ceramic: released calcium ions promote osteogenic differentiation in osteoblastic ROS17/2.8 cells. J Biomed Mater Res. 1999; 47:176–188. PMID: 10449628.
Article20. Yuan H, Fernandes H, Habibovic P, de Boer J, Barradas AM, de Ruiter A, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci U S A. 2010; 107:13614–13619. PMID: 20643969.
Article21. Ripamonti U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 1996; 17:31–35. PMID: 8962945.
Article22. Gosain AK, Song L, Riordan P, Amarante MT, Nagy PG, Wilson CR, et al. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: part I. Plast Reconstr Surg. 2002; 109:619–630. PMID: 11818845.
Article23. Park JW, Jang JH, Bae SR, An CH, Suh JY. Bone formation with various bone graft substitutes in critical-sized rat calvarial defect. Clin Oral Implants Res. 2009; 20:372–378. PMID: 19309771.
Article24. von Arx T, Alsaeed M. The use of regenerative techniques in apical surgery: a literature review. Saudi Dent J. 2011; 23:113–127. PMID: 24151420.
Article25. Deng Y, Zhu X, Yang J, Jiang H, Yan P. The effect of regeneration techniques on periapical surgery with different protocols for different lesion types: a meta-analysis. J Oral Maxillofac Surg. 2016; 74:239–246. PMID: 26546842.
Article26. Alnemer NA, Alquthami H, Alotaibi L. The use of bone graft in the treatment of periapical lesion. Saudi Endod J. 2017; 7:115–118.27. Sreedevi P, Varghese N, Varugheese JM. Prognosis of periapical surgery using bonegrafts: a clinical study. J Conserv Dent. 2011; 14:68–72. PMID: 21691510.
Article28. Taschieri S, Del Fabbro M, Testori T, Saita M, Weinstein R. Efficacy of guided tissue regeneration in the management of through-and-through lesions following surgical endodontics: a preliminary study. Int J Periodontics Restorative Dent. 2008; 28:265–271. PMID: 18605602.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on bioceramics abstracted from fishbone(natural apatite)
- Reconstruction of Hemifacial Atrophy with Lateral Arm Adipofascial Flap and Orthognathic Surgery: A Case Report
- Immediate reconstruction using vertical ramus osteotomy and bone slidng after condylectomy due to osteochondroma: a case report
- Osteochonrdoma Of The Mandibular Condyle: A Case Report
- The reconstruction of posttraumatic craniofacial deformity using modified lefort II and III osteotomy and autogenous iliac bone graft