Cancer Res Treat.
2016 Apr;48(2):658-667. 10.4143/crt.2015.193.
Dental Anomalies as Late Adverse Effect among Young Children Treated for Cancer
- Affiliations
-
- 1Department of Pediatric Dentistry, Oncology, Hematology and Diabetology, Medical University of Åódź, Åódź, Poland. patrycja.proc@umed.lodz.pl
- 2Department of Orthodontics, Oncology, Hematology and Diabetology, Medical University of Åódź, Åódź, Poland.
- 3Department of Pediatrics, Oncology, Hematology and Diabetology, Medical University of Åódź, Åódź, Poland.
- KMID: 2454344
- DOI: http://doi.org/10.4143/crt.2015.193
Abstract
- PURPOSE
The aim of this study was to compare the incidence of dental complications in childhood cancer survivors with that of healthy control subjects, and to determine the possible influence of various factors associated with patient and treatment.
MATERIALS AND METHODS
Sixty-one panoramic radiographs of the dentition of cancer survivors were compared with 521 radiographs of healthy patients at a similar age, between 5 and 18 years. The mean period from termination of therapy was 4.9 years (58.9±34.3 months), and 51 children (83.60%) were under age 5 when therapy began.
RESULTS
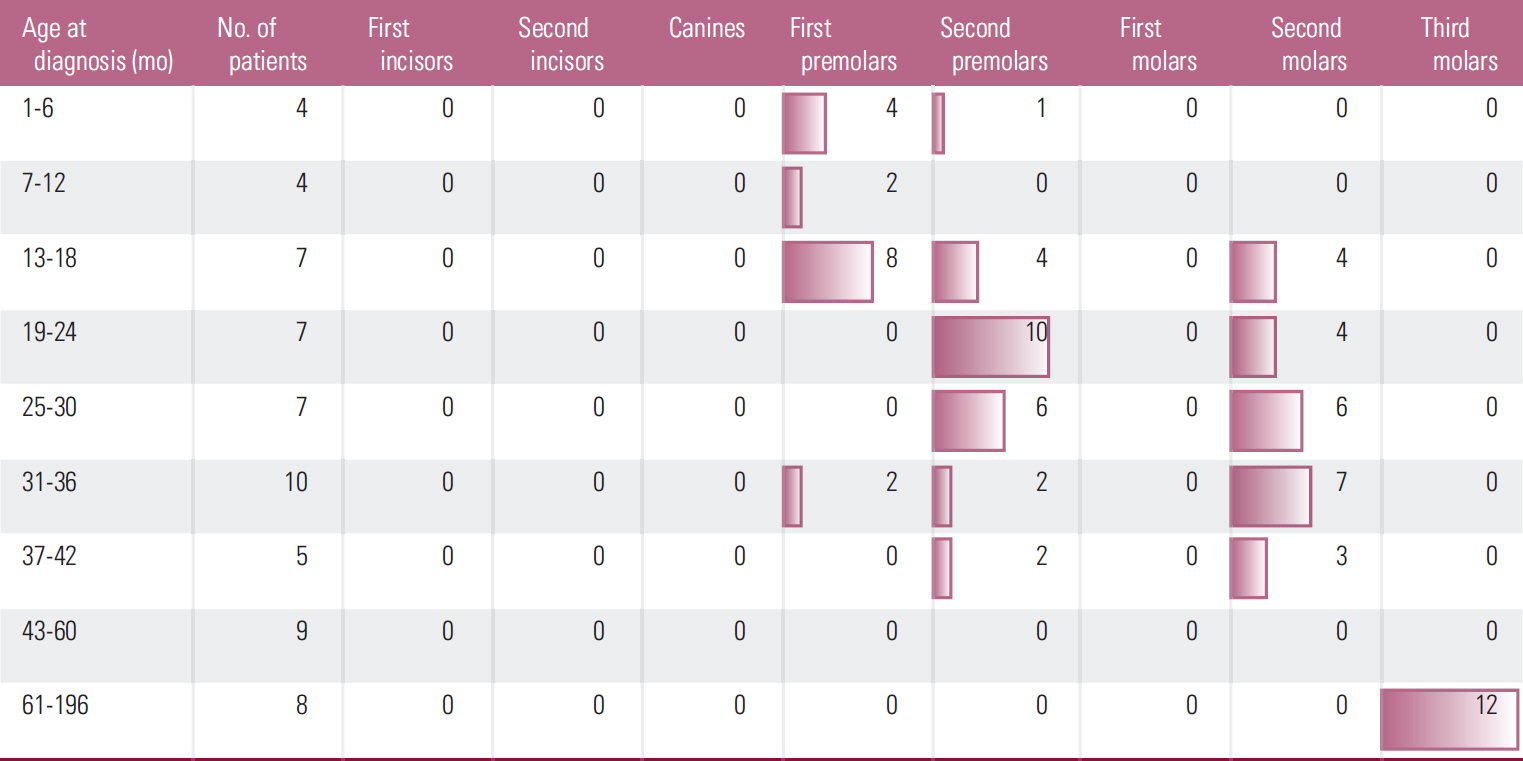
Dental anomalies were found in 38 cancer survivors (62.29%) and 69 control subjects (13.24%) (p < 0.001). Agenesis of teeth was found in 19 cancer patients (31.14%) and in 48 control subjects (9.21%). Microdontic teeth were found in 22 cancer survivors (36.06%) and 15 control subjects (2.87%) (p < 0.001), whereas teeth with short roots were found in seven cancer patients (11.47%) and 15 control subjects (2.87%) (p < 0.01). Dental anomalies in cancer patients were more common in some tooth groups and were not observed in others. The frequency of dental anomalies did not show correlation with age at the beginning or termination or time of therapy.
CONCLUSION
Children under the age of 5 are in a high risk group for dental complications after anticancer treatment. Rudimentary chemotherapy has a considerable impact on the occurrence of dental anomalies.
Keyword
Figure
Cited by 1 articles
-
Clinical Risk Factors Influencing Dental Developmental Disturbances in Childhood Cancer Survivors
Chung-Min Kang, Seung Min Hahn, Hyo Sun Kim, Chuhl Joo Lyu, Jae-Ho Lee, Jinae Lee, Jung Woo Han
Cancer Res Treat. 2018;50(3):926-935. doi: 10.4143/crt.2017.296.
Reference
-
References
1. Geenen MM, Cardous-Ubbink MC, Kremer LC, van den Bos C, van der Pal HJ, Heinen RC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007; 297:2705–15.
Article2. Zubowska M, Wyka K, Fendler W, Mlynarski W, Zalewska-Szewczyk B. Interleukin 18 as a marker of chronic nephropathy in children after anticancer treatment. Dis Markers. 2013; 35:811–8.
Article3. Kaste SC, Goodman P, Leisenring W, Stovall M, Hayashi RJ, Yeazel M, et al. Impact of radiation and chemotherapy on risk of dental abnormalities: a report from the Childhood Cancer Survivor Study. Cancer. 2009; 115:5817–27.4. Dahl JE. Immediate and delayed effects of repeated doxorubicin injections on rat incisor mesenchymal cells. Acta Odontol Scand. 1985; 43:155–62.
Article5. Jaffe N, Toth BB, Hoar RE, Ried HL, Sullivan MP, McNeese MD. Dental and maxillofacial abnormalities in long-term survivors of childhood cancer: effects of treatment with chemotherapy and radiation to the head and neck. Pediatrics. 1984; 73:816–23.
Article6. Karim AC, Woltgens JH, Bervoets TJ, Lyaruu DM, Bronckers AL. Effect of adriamycin on hamster molar tooth development in vitro: 1. Morphological changes. Anat Rec. 1989; 225:318–28.
Article7. Lyaruu DM, van Duin MA, Bervoets TJ, Bronckers AL, Woltgens JH. Daunorubicin-induced pathology in the developing hamster molar tooth germ in vitro. Cancer Detect Prev. 1999; 23:343–50.
Article8. Lyaruu DM, van Duin MA, Bervoets TJ, Woltgens JH, Bronckers AL. Effects of vincristine on the developing hamster tooth germ in vitro. Connect Tissue Res. 1995; 32:281–9.9. Mitomi T, Kawano Y, Kinoshita-Kawano S. Effect of the antineoplastic agent busulfan on rat molar root development. Arch Oral Biol. 2014; 59:47–59.
Article10. Nasman M, Hammarstrom L. Influence of the antineoplastic agent cyclophosphamide on dental development in rat molars. Acta Odontol Scand. 1996; 54:287–94.11. Holtta P, Alaluusua S, Saarinen-Pihkala UM, Peltola J, Hovi L. Agenesis and microdontia of permanent teeth as late adverse effects after stem cell transplantation in young children. Cancer. 2005; 103:181–90.
Article12. Pedersen LB, Clausen N, Schroder H, Schmidt M, Poulsen S. Microdontia and hypodontia of premolars and permanent molars in childhood cancer survivors after chemotherapy. Int J Paediatr Dent. 2012; 22:239–43.
Article13. Holtta P, Hovi L, Saarinen-Pihkala UM, Peltola J, Alaluusua S. Disturbed root development of permanent teeth after pediatric stem cell transplantation. Dental root development after SCT. Cancer. 2005; 103:1484–93.14. Cubukcu CE, Sevinir B, Ercan I. Disturbed dental development of permanent teeth in children with solid tumors and lymphomas. Pediatr Blood Cancer. 2012; 58:80–4.
Article15. Polder BJ, Van't Hof MA, Van der Linden FP, Kuijpers-Jagtman AM. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent Oral Epidemiol. 2004; 32:217–26.
Article16. Backman B, Wahlin YB. Variations in number and morphology of permanent teeth in 7-year-old Swedish children. Int J Paediatr Dent. 2001; 11:11–7.17. Brook AH. Multilevel complex interactions between genetic, epigenetic and environmental factors in the aetiology of anomalies of dental development. Arch Oral Biol. 2009; 54 Suppl 1:S3–17.
Article18. Schalk van der Weide Y. Oligodontia: a clinical, radiographic and genetic evaluation [thesis]. Ultrecht: Ultrecht University;1992.19. Pawlowska E, Janik-Papis K, Poplawski T, Blasiak J, Szczepanska J. Mutations in the PAX9 gene in sporadic oligodontia. Orthod Craniofac Res. 2010; 13:142–52.
Article20. Ramazanzadeh BA, Ahrari F, Hajian S. Evaluation of tooth size in patients with congenitally-missing teeth. J Dent Res Dent Clin Dent Prospects. 2013; 7:36–41.21. Tan SP, van Wijk AJ, Prahl-Andersen B. Severe hypodontia: identifying patterns of human tooth agenesis. Eur J Orthod. 2011; 33:150–4.
Article22. Logan WH, Kronfeld R. Development of the human jaws and surrounding structures from birth to the age of fifteen years. J Am Dent Assoc. 1933; 20:379–428.
Article23. Apajalahti S, Holtta P, Turtola L, Pirinen S. Prevalence of short-root anomaly in healthy young adults. Acta Odontol Scand. 2002; 60:56–9.
Article24. Shifman A, Chanannel I. Prevalence of taurodontism found in radiographic dental examination of 1,200 young adult Israeli patients. Community Dent Oral Epidemiol. 1978; 6:200–3.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of Dental Anomalies in Patients with Non-syndromic Cleft Lip with or without Cleft Palate
- Parenting experiences of mothers of moderate-to-late preterm children in South Korea: a qualitative study
- Hyperfractionated radiotherapy for re-irradiation of recurrent esophageal cancer
- Outpatient General Anesthesia for Mentally and Physically Handicapped Children Undergoing Extensive Dental Treatment
- Trends and Characteristics of Mortality Associated with Congenital Anomalies in Korean Children under 5 Years of Age