Anat Cell Biol.
2018 Sep;51(3):150-157. 10.5115/acb.2018.51.3.150.
Development of the pulmonary pleura with special reference to the lung surface morphology: a study using human fetuses
- Affiliations
-
- 1Department of Anatomy, Tokyo Dental College, Tokyo, Japan. yamamotomasahito@tdc.ac.jp
- 2Institute of Anatomy and Cell Biology, School of Medicine, Georg-August-Universität Gőttingen, Gőttingen, Germany.
- 3Department of Anatomy, Akita University School of Medicine, Akita, Japan.
- 4Division of Internal Medicine, Iwamizawa Asuka Hospital, Iwamizawa, Japan.
- 5Department of Anatomy and Human Embryology, Institute of Embryology, Faculty of Medicine, Complutense University, Madrid, Spain.
- KMID: 2447007
- DOI: http://doi.org/10.5115/acb.2018.51.3.150
Abstract
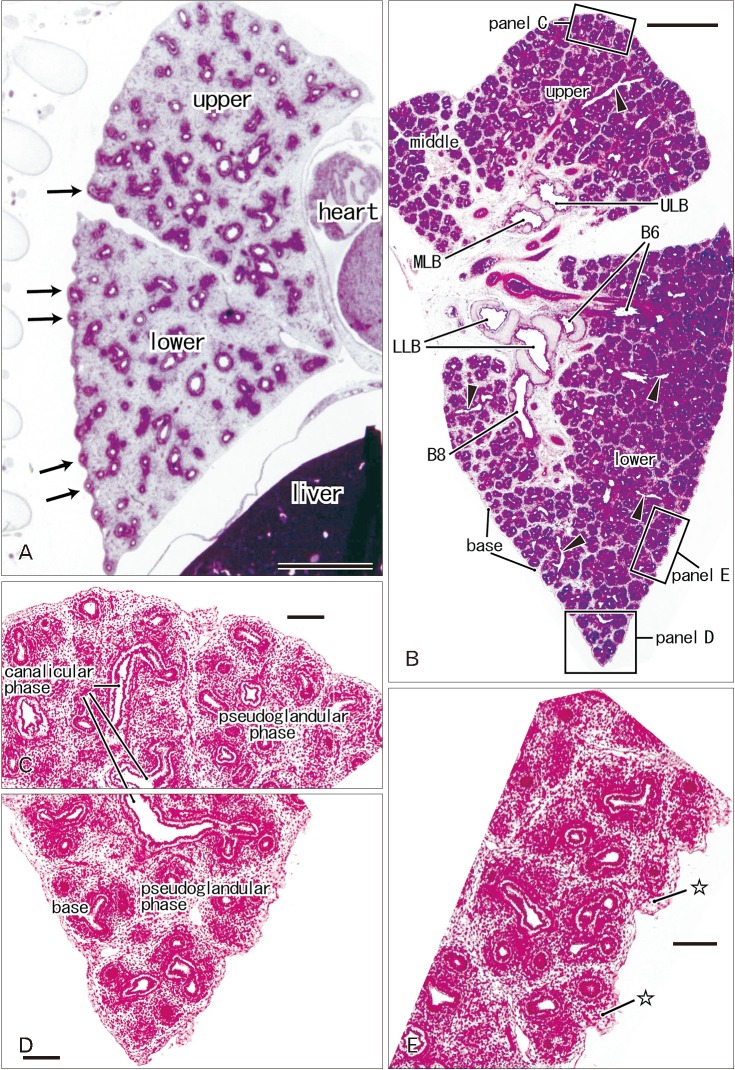
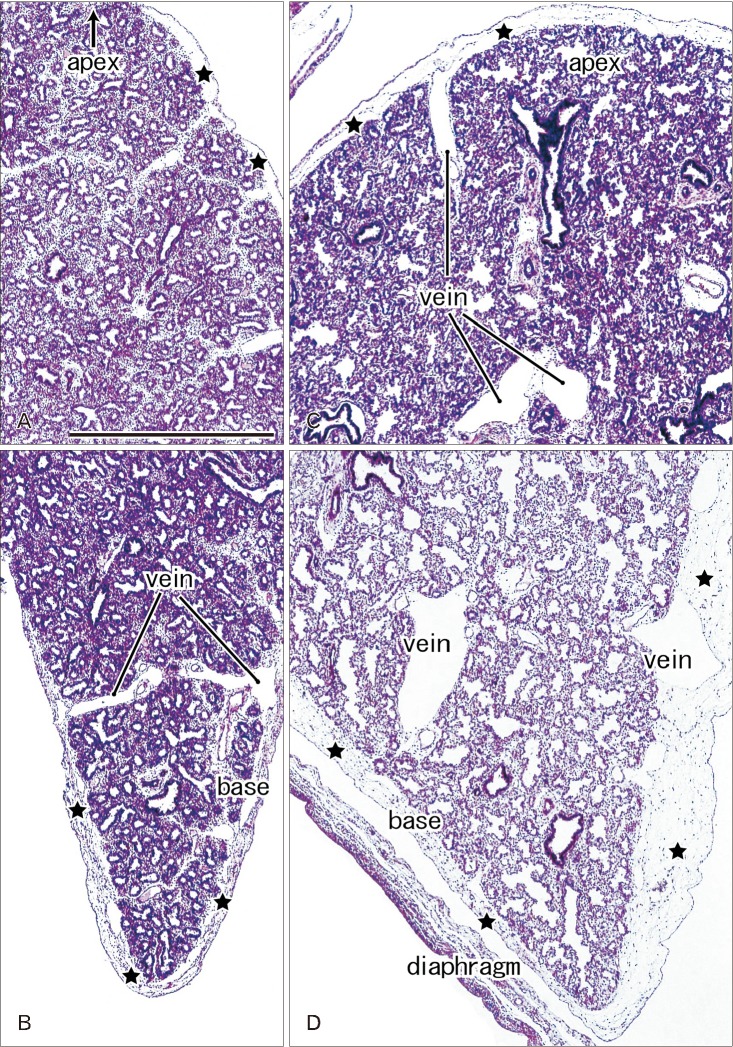
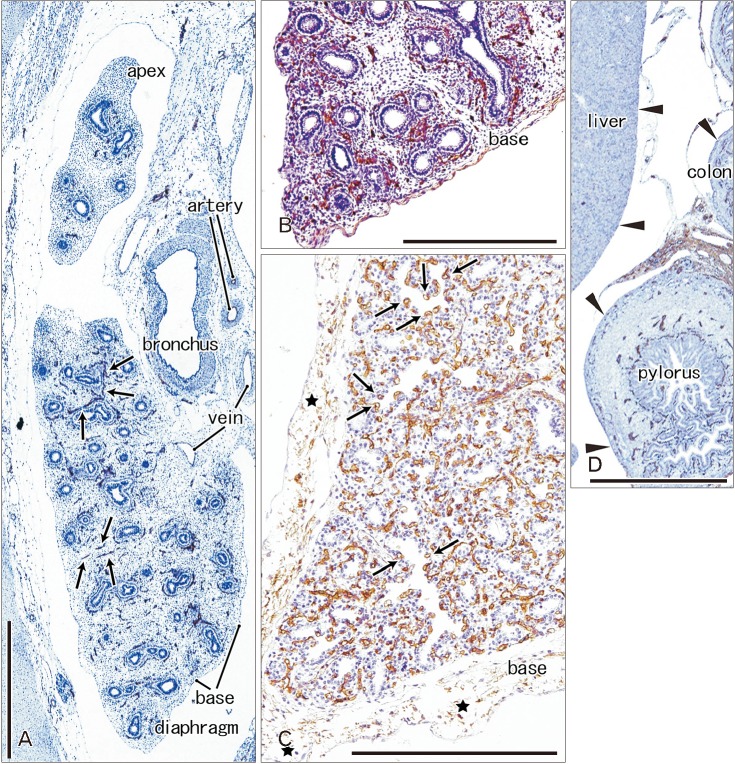
- In and after the third trimester, the lung surface is likely to become smooth to facilitate respiratory movements. However, there are no detailed descriptions as to when and how the lung surface becomes regular. According to our observations of 33 fetuses at 9-16 weeks of gestation (crown-rump length [CRL], 39-125 mm), the lung surface, especially its lateral (costal) surface, was comparatively rough due to rapid branching and outward growing of bronchioli at the pseudoglandular phase of lung development. The pulmonary pleura was thin and, beneath the surface mesothelium, no or little mesenchymal tissue was detectable. Veins and lymphatic vessels reached the lung surface until 9 weeks and 16 weeks, respectively. In contrast, in 8 fetuses at 26-34 weeks of gestation (CRL, 210-290 mm), the lung surface was almost smooth because, instead of bronchioli, the developing alveoli faced the external surfaces of the lung. Moreover, the submesothelial tissue became thick due to large numbers of dilated veins connected to deep intersegmental veins. CD34-positive, multilayered fibrous tissue was also evident beneath the mesothelium in these stages. The submesothelial tissue was much thicker at the basal and mediastinal surfaces compared to apical and costal surfaces. Overall, rather than by a mechanical stress from the thoracic wall and diaphragm, a smooth lung surface seemed to be established largely by the thick submesothelial tissue including veins and lymphatic vessels until 26 weeks.
MeSH Terms
Figure
Reference
-
1. Finley DJ, Rusch VW. Anatomy of the pleura. Thorac Surg Clin. 2011; 21:157–163. PMID: 21477764.2. O'Rahilly R, Müller F. Human embryology and teratology. 2nd ed. New York: Wiley-Liss;1996. p. 265–271.3. Hayashi S, Fukuzawa Y, Rodríguez-Vázquez JF, Cho BH, Verdugo-López S, Murakami G, Nakano T. Pleuroperitoneal canal closure and the fetal adrenal gland. Anat Rec (Hoboken). 2011; 294:633–644. PMID: 21370493.4. Abe S, Suzuki M, Cho KH, Murakami G, Cho BH, Ide Y. CD34-positive developing vessels and other structures in human fetuses: an immunohistochemical study. Surg Radiol Anat. 2011; 33:919–927. PMID: 21789504.5. Katori Y, Kiyokawa H, Kawase T, Murakami G, Cho BH. CD34-positive primitive vessels and other structures in human fetuses: an immunohistochemical study. Acta Otolaryngol. 2011; 131:1086–1090. PMID: 21651317.6. Wilting J, Papoutsi M, Christ B, Nicolaides KH, von Kaisenberg CS, Borges J, Stark GB, Alitalo K, Tomarev SI, Niemeyer C, Rössler J. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J. 2002; 16:1271–1273. PMID: 12060670.7. Hasselhof V, Sperling A, Buttler K, Ströbel P, Becker J, Aung T, Felmerer G, Wilting J. Morphological and molecular characterization of human dermal lymphatic collectors. PLoS One. 2016; 11:e0164964. PMID: 27764183.8. Kinoshita H, Umezawa T, Omine Y, Kasahara M, Rodríguez-Vázquez JF, Murakami G, Abe S. Distribution of elastic fibers in the head and neck: a histological study using late-stage human fetuses. Anat Cell Biol. 2013; 46:39–48. PMID: 23560235.9. Motohashi O, Suzuki M, Shida N, Umezawa K, Ohtoh T, Sakurai Y, Yoshimoto T. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunhistochemicalstudy. Acta Neurochir (Wien). 1995; 136:88–91. PMID: 8748833.10. Hayashi T, Kumasaka T, Mitani K, Yao T, Suda K, Seyama K. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod Pathol. 2010; 23:1251–1260. PMID: 20526286.11. Riquet M. Anatomic basis of lymphatic spread from carcinoma of the lung to the mediastinum: surgical and prognostic implications. Surg Radiol Anat. 1993; 15:271–277. PMID: 8128334.12. Topol M, Masłoń A. The problem of direct lymph drainage of the bronchopulmonary segments into the mediastinal and hilar lymph nodes. Clin Anat. 2009; 22:509–516. PMID: 19306320.13. Skandalakis JE, Gray SW, Symbas PN. The trachea and lungs. In : Skandalakis JE, Gray SW, editors. Embryology for Surgeons. 2nd ed. Baltimore, MD: Williams & Wilkins;1972. p. 414–450.14. Virtanen I, Laitinen A, Tani T, Pääkkö P, Laitinen LA, Burgeson RE, Lehto VP. Differential expression of laminins and their integrin receptors in developing and adult human lung. Am J Respir Cell Mol Biol. 1996; 15:184–196. PMID: 8703474.15. Arai H, Hirano H, Mushiake S, Nakayama M, Takada G, Sekiguchi K. Loss of EDB+ fibronectin isoform is associated with differentiation of alveolar epithelial cells in human fetal lung. Am J Pathol. 1997; 151:403–412. PMID: 9250153.16. Wright C, Strauss S, Toole K, Burt AD, Robson SC. Composition of the pulmonary interstitium during normal development of the human fetus. Pediatr Dev Pathol. 1999; 2:424–431. PMID: 10441619.17. Lambropoulou M, Limberis V, Koutlaki N, Simopoulou M, Ntanovasilis D, Vandoros GP, Tatsidou P, Kekou I, Koutsikogianni I, Papadopoulos N. Differential expression of tenascin-C in the developing human lung: an immunohistochemical study. Clin Exp Med. 2009; 9:333–338. PMID: 19626416.18. Jin ZW, Nakamura T, Yu HC, Kimura W, Murakami G, Cho BH. Fetal anatomy of peripheral lymphatic vessels: a D2-40 immunohistochemical study using an 18-week human fetus (CRL 155 mm). J Anat. 2010; 216:671–682. PMID: 20408907.19. Kim JH, Han EH, Jin ZW, Lee HK, Fujimiya M, Murakami G, Cho BH. Fetal topographical anatomy of the upper abdominal lymphatics: its specific features in comparison with other abdominopelvic regions. Anat Rec (Hoboken). 2012; 295:91–104. PMID: 22144396.