Korean J Radiol.
2019 May;20(5):759-772. 10.3348/kjr.2018.0515.
A Prospective Study on the Value of Ultrasound Microflow Assessment to Distinguish Malignant from Benign Solid Breast Masses: Association between Ultrasound Parameters and Histologic Microvessel Densities
- Affiliations
-
- 1Department of Radiology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. seoboky@korea.ac.kr
- 2Department of Radiology, Bundang CHA Medical Center, CHA University, Seongnam, Korea.
- 3Department of Radiology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- 4Department of Radiology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 5Department of Pathology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
- 6Medical Science Research Center, Korea University Ansan Hospital, Ansan, Korea.
- 7Division of Breast Endocrine Surgery, Department of General Surgery, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
- KMID: 2442710
- DOI: http://doi.org/10.3348/kjr.2018.0515
Abstract
OBJECTIVE
To investigate the value of ultrasound (US) microflow assessment in distinguishing malignant from benign solid breast masses as well as the association between US parameters and histologic microvessel density (MVD).
MATERIALS AND METHODS
Ninety-eight breast masses (57 benign and 41 malignant) were examined using Superb Microvascular Imaging (SMI) and contrast-enhanced US (CEUS) before biopsy. Two radiologists evaluated the quantitative and qualitative vascular parameters on SMI (vascular index, morphology, distribution, and penetration) and CEUS (time-intensity curve analysis and enhancement characteristics). US parameters were compared between benign and malignant masses and the diagnostic performance was compared between SMI and CEUS. Subgroup analysis was performed according to lesion size. The effect of vascular parameters on downgrading Breast Imaging Reporting and Data System (BI-RADS) category 4A masses was evaluated. The association between histologic MVD and US parameters was analyzed.
RESULTS
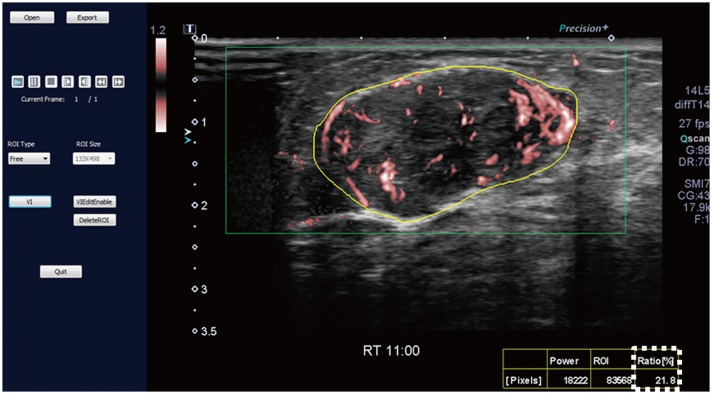
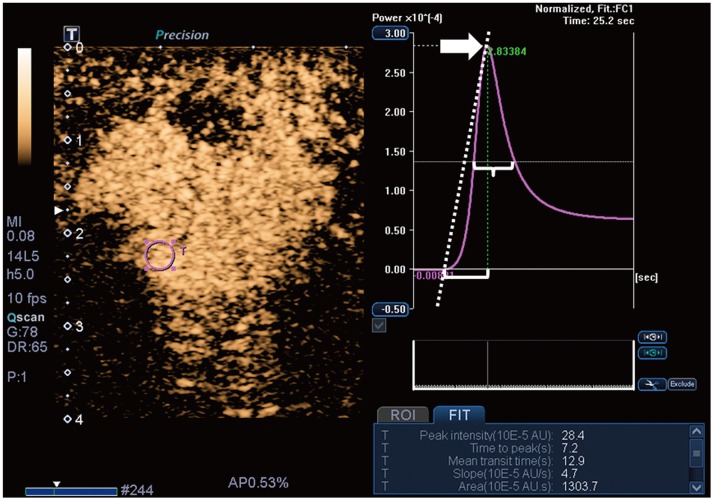
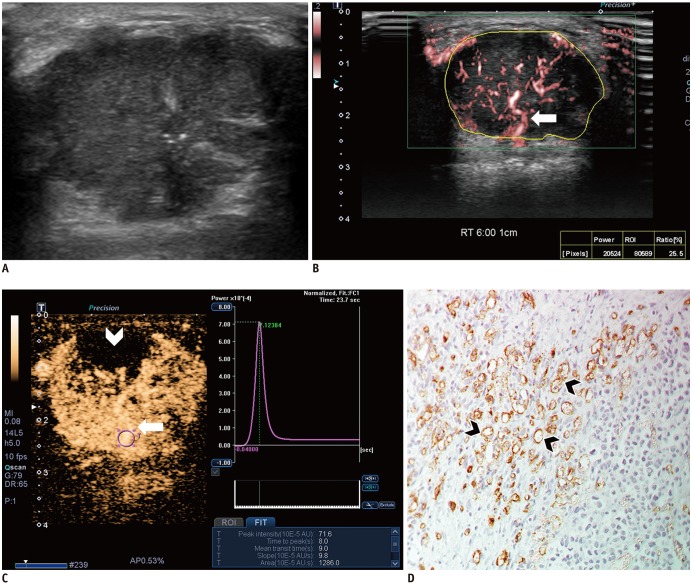
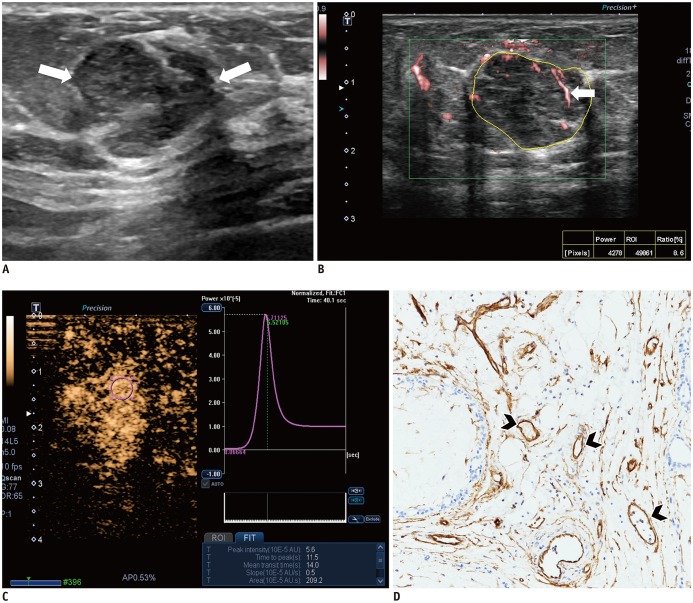
Malignant masses were associated with a higher vascular index (15.1 ± 7.3 vs. 5.9 ± 5.6), complex vessel morphology (82.9% vs. 42.1%), central vascularity (95.1% vs. 59.6%), penetrating vessels (80.5% vs. 31.6%) on SMI (all, p < 0.001), as well as higher peak intensity (37.1 ± 25.7 vs. 17.0 ± 15.8, p < 0.001), slope (10.6 ± 11.2 vs. 3.9 ± 4.2, p = 0.001), area (1035.7 ± 726.9 vs. 458.2 ± 410.2, p < 0.001), hyperenhancement (95.1% vs. 70.2%, p = 0.005), centripetal enhancement (70.7% vs. 45.6%, p = 0.023), penetrating vessels (65.9% vs. 22.8%, p < 0.001), and perfusion defects (31.7% vs. 3.5%, p < 0.001) on CEUS (p ≤ 0.023). The areas under the receiver operating characteristic curve (AUCs) of SMI and CEUS were 0.853 and 0.841, respectively (p = 0.803). In 19 masses measuring < 10 mm, central vascularity on SMI was associated with malignancy (100% vs. 38.5%, p = 0.018). Considering all benign SMI parameters on the BI-RADS assessment, unnecessary biopsies could be avoided in 12 category 4A masses with improved AUCs (0.500 vs. 0.605, p < 0.001). US vascular parameters associated with malignancy showed higher MVD (p ≤ 0.016). MVD was higher in malignant masses than in benign masses, and malignant masses negative for estrogen receptor or positive for Ki67 had higher MVD (p < 0.05).
CONCLUSION
US microflow assessment using SMI and CEUS is valuable in distinguishing malignant from benign solid breast masses, and US vascular parameters are associated with histologic MVD.
Keyword
MeSH Terms
Figure
Reference
-
1. Yadav L, Puri N, Rastogi V, Satpute P, Sharma V. Tumour angiogenesis and angiogenic inhibitors: a review. J Clin Diagn Res. 2015; 9:XE01–XE05.
Article2. Folkman J. Angiogenesis and breast cancer. J Clin Oncol. 1994; 12:441–443. PMID: 7509850.
Article3. Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol. 2005; 23:1782–1790. PMID: 15755986.
Article4. Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004; 64:2941–2955. PMID: 15126324.5. Lee SW, Choi HY, Baek SY, Lim SM. Role of color and power doppler imaging in differentiating between malignant and benign solid breast masses. J Clin Ultrasound. 2002; 30:459–464. PMID: 12242733.
Article6. Kook SH, Park HW, Lee YR, Lee YU, Pae WK, Park YL. Evaluation of solid breast lesions with power Doppler sonography. J Clin Ultrasound. 1999; 27:231–237. PMID: 10355886.
Article7. Giuseppetti GM, Baldassarre S, Marconi E. Color Doppler sonography. Eur J Radiol. 1998; 27(Suppl 2):S254–S258. PMID: 9652531.
Article8. Schroeder RJ, Bostanjoglo M, Rademaker J, Maeurer J, Felix R. Role of power Doppler techniques and ultrasound contrast enhancement in the differential diagnosis of focal breast lesions. Eur Radiol. 2003; 13:68–79. PMID: 12541112.
Article9. Lee WJ, Chu JS, Houng SJ, Chung MF, Wang SM, Chen KM. Breast cancer angiogenesis: a quantitative morphologic and Doppler imaging study. Ann Surg Oncol. 1995; 2:246–251. PMID: 7543800.
Article10. Yang WT, Tse GM, Lam PK, Metreweli C, Chang J. Correlation between color power Doppler sonographic measurement of breast tumor vasculature and immunohistochemical analysis of microvessel density for the quantitation of angiogenesis. J Ultrasound Med. 2002; 21:1227–1235. PMID: 12418764.
Article11. Ma Y, Li G, Li J, Ren WD. The Diagnostic value of superb microvascular imaging (SMI) in detecting blood flow signals of breast lesions: a preliminary study comparing SMI to color Doppler flow imaging. Medicine (Baltimore). 2015; 94:e1502. PMID: 26356718.12. Park AY, Seo BK, Cha SH, Yeom SK, Lee SW, Chung HH. An innovative ultrasound technique for evaluation of tumor vascularity in breast cancers: superb micro-vascular imaging. J Breast Cancer. 2016; 19:210–213. PMID: 27382399.
Article13. Yongfeng Z, Ping Z, Wengang L, Yang S, Shuangming T. Application of a novel microvascular imaging technique in breast lesion evaluation. Ultrasound Med Biol. 2016; 42:2097–2105. PMID: 27321174.
Article14. Zhan J, Diao XH, Jin JM, Chen L, Chen Y. Superb microvascular imaging-a new vascular detecting ultrasonographic technique for avascular breast masses: a preliminary study. Eur J Radiol. 2016; 85:915–921. PMID: 27130051.
Article15. Park AY, Seo BK, Woo OH, Jung KS, Cho KR, Park EK, et al. The utility of ultrasound superb microvascular imaging for evaluation of breast tumour vascularity: comparison with colour and power Doppler imaging regarding diagnostic performance. Clin Radiol. 2018; 73:304–311. PMID: 29122223.
Article16. Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography. 2015; 34:3–18. PMID: 25342120.
Article17. Du J, Wang L, Wan CF, Hua J, Fang H, Chen J, et al. Differentiating benign from malignant solid breast lesions: combined utility of conventional ultrasound and contrast-enhanced ultrasound in comparison with magnetic resonance imaging. Eur J Radiol. 2012; 81:3890–3899. PMID: 23062280.
Article18. Zhao H, Xu R, Ouyang Q, Chen L, Dong B, Huihua Y. Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions. Eur J Radiol. 2010; 73:288–293. PMID: 19559551.
Article19. Ricci P, Cantisani V, Ballesio L, Pagliara E, Sallusti E, Drudi FM, et al. Benign and malignant breast lesions: efficacy of real time contrast-enhanced ultrasound vs. magnetic resonance imaging. Ultraschall Med. 2007; 28:57–62. PMID: 17304413.
Article20. Liu H, Jiang YX, Liu JB, Zhu QL, Sun Q. Evaluation of breast lesions with contrast-enhanced ultrasound using the microvascular imaging technique: initial observations. Breast. 2008; 17:532–539. PMID: 18534851.
Article21. Drudi FM, Cantisani V, Gnecchi M, Malpassini F, Di Leo N, de Felice C. Contrast-enhanced ultrasound examination of the breast: a literature review. Ultraschall Med. 2012; 33:E1–E7. PMID: 22623129.
Article22. Mendelson EB, Böhm-Vélez M, Berg WA, Whitman GJ, Feldman MI, Madjar H, et al. ACR BI-RADS ultrasound. In : D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, editors. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology;2013. p. 1–173.23. Wan CF, Du J, Fang H, Li FH, Zhu JS, Liu Q. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology. 2012; 262:450–459. PMID: 22282183.
Article24. Wan C, Du J, Fang H, Li F, Wang L. Evaluation of breast lesions by contrast enhanced ultrasound: qualitative and quantitative analysis. Eur J Radiol. 2012; 81:e444–e450. PMID: 21612882.
Article25. Lassau N, Chami L, Benatsou B, Peronneau P, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol. 2007; 17(Suppl 6):F89–F98. PMID: 18376462.
Article26. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon: International Agency for Research on Cancer;2012.27. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010; 134:e48–e72. PMID: 20586616.28. Pitre-Champagnat S, Leguerney I, Bosq J, Peronneau P, Kiessling F, Calmels L, et al. Dynamic contrast-enhanced ultrasound parametric maps to evaluate intratumoral vascularization. Invest Radiol. 2015; 50:212–217. PMID: 25275834.
Article29. Du J, Li FH, Fang H, Xia JG, Zhu CX. Correlation of real-time gray scale contrast-enhanced ultrasonography with microvessel density and vascular endothelial growth factor expression for assessment of angiogenesis in breast lesions. J Ultrasound Med. 2008; 27:821–831. PMID: 18499842.
Article30. Xiao XY, Chen X, Guan XF, Wu H, Qin W, Luo BM. Superb microvascular imaging in diagnosis of breast lesions: a comparative study with contrast-enhanced ultrasonographic microvascular imaging. Br J Radiol. 2016; 89:20160546. PMID: 27529640.
Article31. Chao TC, Lo YF, Chen SC, Chen MF. Color Doppler ultrasound in benign and malignant breast tumors. Breast Cancer Res Treat. 1999; 57:193–199. PMID: 10598046.
Article32. Miyamoto Y, Ito T, Takada E, Omoto K, Hirai T, Moriyasu F. Efficacy of sonazoid (perflubutane) for contrast-enhanced ultrasound in the differentiation of focal breast lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2014; 202:W400–W407. PMID: 24660739.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of Contrast Enhanced Power Doppler Sonographic Findings with Histologic Microvessel Density in the Breast Mass
- Evaluation of Solid Breast Masses with Power Doppler Ultrasound
- The diagnostic value of ultrasound-guided fine-needle aspiration biopsy in breast masses
- Complex Cysts of the Breast: Analysis using Sonographic Breast Imaging Reporting and Data System
- Detection Rate of Breast Lesion on Mammogram Shown on Breast Sonogram