J Periodontal Implant Sci.
2019 Feb;49(1):14-24. 10.5051/jpis.2019.49.1.14.
Influence of wound closure on volume stability with the application of different GBR materials: an in vitro cone-beam computed tomographic study
- Affiliations
-
- 1Clinic of Fixed and Removable Prosthodontics and Dental Material Science, Center of Dental Medicine, University of Zurich, Zurich, Switzerland. nadja.naenni@zzm.uzh.ch
- KMID: 2440174
- DOI: http://doi.org/10.5051/jpis.2019.49.1.14
Abstract
- PURPOSE
To assess the influence of using different combinations of guided bone regeneration (GBR) materials on volume changes after wound closure at peri-implant dehiscence defects.
METHODS
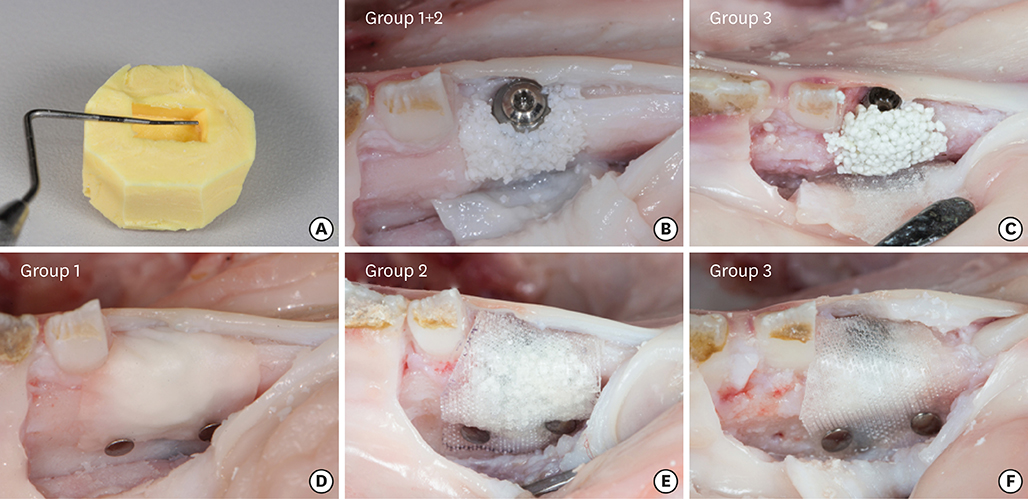
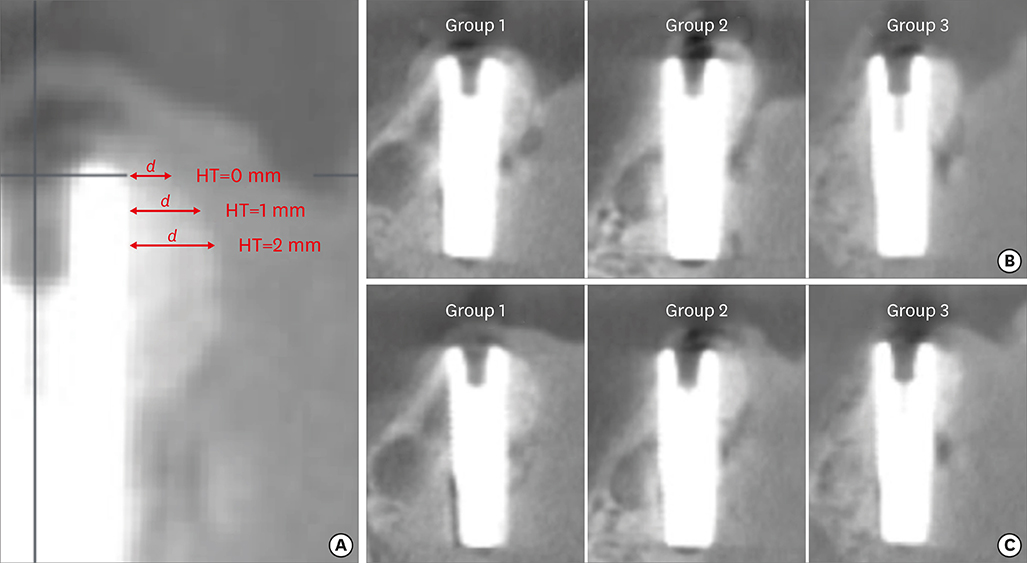
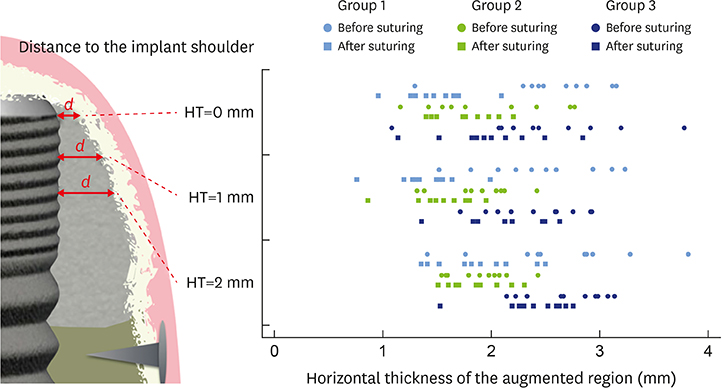
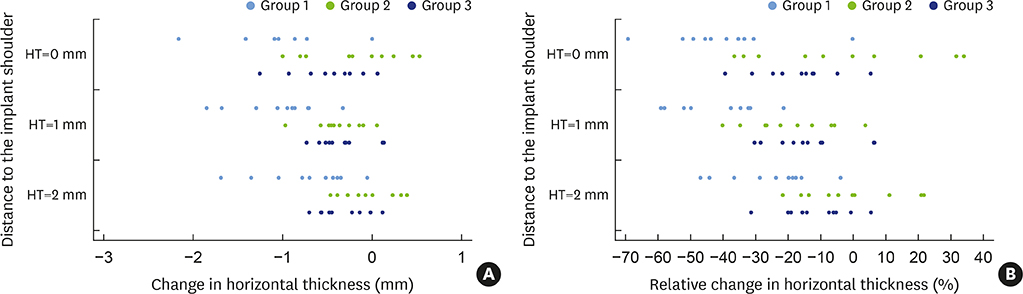
In 5 pig mandibles, standardized bone defects were created and implants were centrally placed. The defects were augmented using different combinations of GBR materials: xenogeneic granulate and collagen membrane (group 1, n=10), xenogeneic granulate and alloplastic membrane (group 2, n=10), alloplastic granulates and alloplastic membrane (group 3, n=10). The horizontal thickness was assessed using cone-beam computed tomography before and after suturing. Measurements were performed at the implant shoulder (HT0) and at 1 mm (HT1) and 2 mm (HT2) below. The data were statistically analysed using the Wilcoxon signed-rank test to evaluate within-group differences. Bonferroni correction was applied when calculating statistical significance between the groups.
RESULTS
The mean horizontal thickness before suturing was 2.55±0.53 mm (group 1), 1.94±0.56 mm (group 2), and 2.49±0.73 mm (group 3). Post-suturing, the values were 1.47±0.31 mm (group 1), 1.77±0.27 mm (group 2), and 2.00±0.48 mm (group 3). All groups demonstrated a loss of horizontal dimension. Intragroup changes exhibited significant differences in group 1 (P < 0.001) and group 3 (P < 0.01). Intergroup comparisons revealed statistically significant differences of the relative changes between groups 1 and 2 (P=0.033) and groups 1 and 3 (P=0.015).
CONCLUSIONS
Volume change after wound closure was minimized by using an alloplastic membrane. The stability of the augmented horizontal thickness was most ensured by using this type of membrane irrespective of the bone substitute material used for membrane support.
Keyword
MeSH Terms
Figure
Reference
-
1. Hämmerle CH, Karring T. Guided bone regeneration at oral implant sites. Periodontol 2000. 1998; 17:151–175.
Article2. Bornstein MM, Halbritter S, Harnisch H, Weber HP, Buser D. A retrospective analysis of patients referred for implant placement to a specialty clinic: indications, surgical procedures, and early failures. Int J Oral Maxillofac Implants. 2008; 23:1109–1116.3. Benic GI, Hämmerle CH. Horizontal bone augmentation by means of guided bone regeneration. Periodontol 2000. 2014; 66:13–40.
Article4. Chiapasco M, Casentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants. 2009; 24:Suppl. 237–259.5. Donos N, Mardas N, Chadha V. Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J Clin Periodontol. 2008; 35:173–202.
Article6. Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration--animal and human studies. Periodontol 2000. 1993; 1:26–35.
Article7. Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982; 9:290–296.
Article8. Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984; 11:494–503.
Article9. Dahlin C, Lekholm U, Becker W, Becker B, Higuchi K, Callens A, et al. Treatment of fenestration and dehiscence bone defects around oral implants using the guided tissue regeneration technique: a prospective multicenter study. Int J Oral Maxillofac Implants. 1995; 10:312–318.10. Scantlebury TV. 1982–1992: a decade of technology development for guided tissue regeneration. J Periodontol. 1993; 64:1129–1137.
Article11. Jung RE, Fenner N, Hämmerle CH, Zitzmann NU. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin Oral Implants Res. 2013; 24:1065–1073.
Article12. Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007; 22:Suppl. 49–70.13. Zitzmann NU, Naef R, Schärer P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int J Oral Maxillofac Implants. 1997; 12:844–852.14. Moses O, Pitaru S, Artzi Z, Nemcovsky CE. Healing of dehiscence-type defects in implants placed together with different barrier membranes: a comparative clinical study. Clin Oral Implants Res. 2005; 16:210–219.
Article15. Naenni N, Schneider D, Jung RE, Husler J, Hammerle CH, Thoma DS. Randomized clinical study assessing two membranes for guided bone regeneration of peri-implant bone defects: clinical and histological outcomes at 6 months. Clin Oral Implants Res. 2017; 28:1309–1317.
Article16. Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, Becker J. Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res. 2005; 16:369–378.
Article17. Lundgren AK, Sennerby L, Lundgren D, Taylor A, Gottlow J, Nyman S. Bone augmentation at titanium implants using autologous bone grafts and a bioresorbable barrier. An experimental study in the rabbit tibia. Clin Oral Implants Res. 1997; 8:82–89.
Article18. von Arx T, Broggini N, Jensen SS, Bornstein MM, Schenk RK, Buser D. Membrane durability and tissue response of different bioresorbable barrier membranes: a histologic study in the rabbit calvarium. Int J Oral Maxillofac Implants. 2005; 20:843–853.19. Strietzel FP, Khongkhunthian P, Khattiya R, Patchanee P, Reichart PA. Healing pattern of bone defects covered by different membrane types--a histologic study in the porcine mandible. J Biomed Mater Res B Appl Biomater. 2006; 78:35–46.
Article20. Mir-Mari J, Wui H, Jung RE, Hämmerle CH, Benic GI. Influence of blinded wound closure on the volume stability of different GBR materials: an in vitro cone-beam computed tomographic examination. Clin Oral Implants Res. 2016; 27:258–265.
Article21. Mir-Mari J, Benic GI, Valmaseda-Castellón E, Hämmerle CH, Jung RE. Influence of wound closure on the volume stability of particulate and non-particulate GBR materials: an in vitro cone-beam computed tomographic examination. Part II. Clin Oral Implants Res. 2017; 28:631–639.
Article22. Friedmann A, Strietzel FP, Maretzki B, Pitaru S, Bernimoulin JP. Histological assessment of augmented jaw bone utilizing a new collagen barrier membrane compared to a standard barrier membrane to protect a granular bone substitute material. Clin Oral Implants Res. 2002; 13:587–594.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between cone-beam computed tomographic findings and the apnea-hypopnea index in obstructive sleep apnea patients: A cross-sectional study
- Detection of maxillary second molar with two palatal roots using cone beam computed tomography: a case report
- A rare case of dilated invaginated odontome with talon cusp in a permanent maxillary central incisor diagnosed by cone beam computed tomography
- Management of root canal perforation by using cone-beam computed tomography
- Cone-beam computed tomographic imaging of silent sinus syndrome: A case series and a literature review