Pediatr Infect Vaccine.
2018 Apr;25(1):17-25. 10.14776/piv.2018.25.1.17.
A Multicenter Survey on the Current Status of Pediatric Blood Cultures in Korea
- Affiliations
-
- 1Department of Pediatrics, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, the Republic of Korea. chieunoh@kosin.ac.kr
- KMID: 2438750
- DOI: http://doi.org/10.14776/piv.2018.25.1.17
Abstract
- PURPOSE
Blood culture is an essential diagnostic tool and requires clear indications, proper techniques, and quality control. We aimed to investigate whether blood cultures in children are appropriate for indications, are performed correctly, and receive proper quality control.
METHODS
We conducted an online survey targeting pediatric infectious diseases (ID) specialists working in general hospitals and neonatologists (Neo) working at hospitals operating a neonatal intensive care unit in Korea.
RESULTS
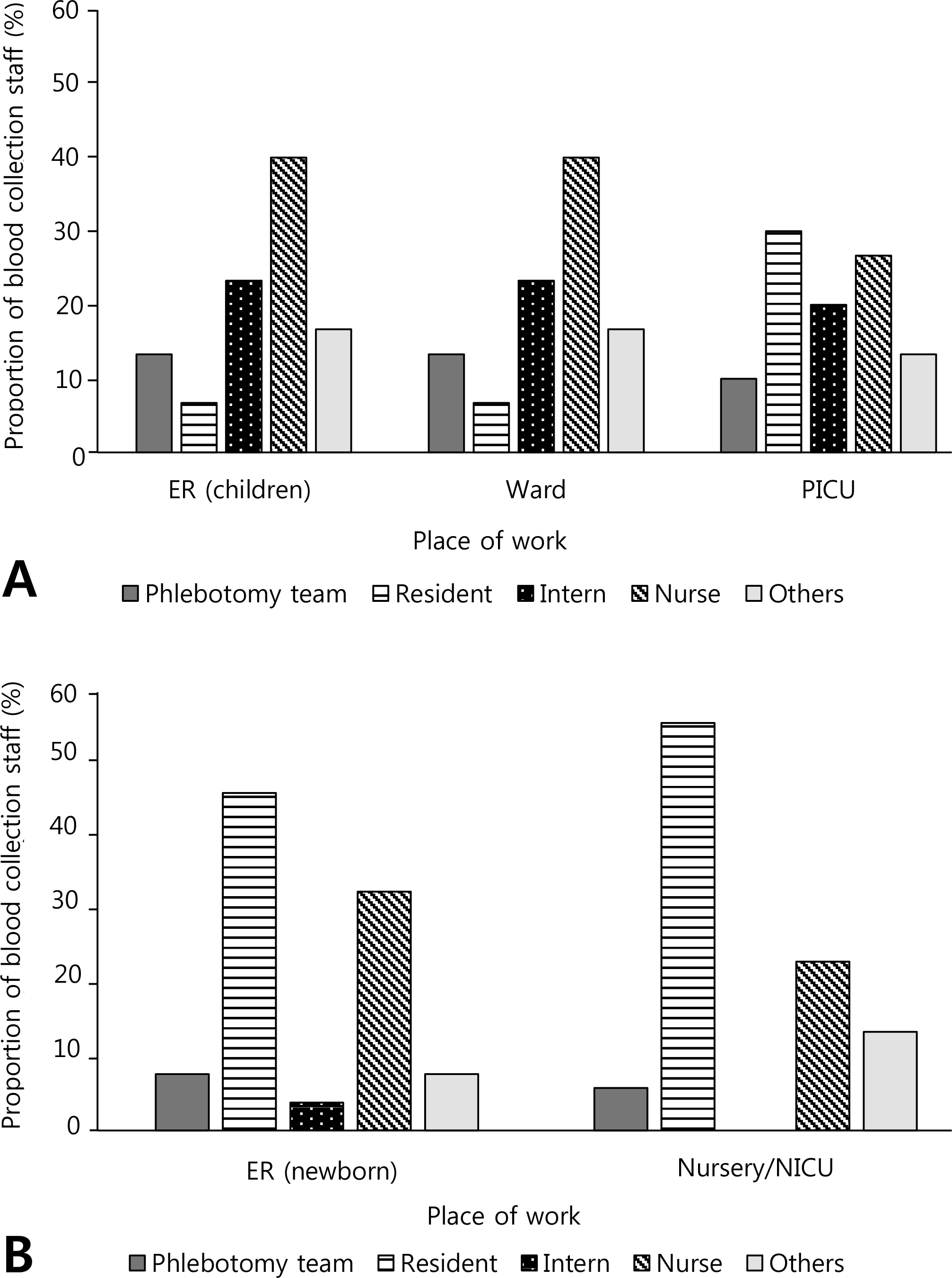
Approximately 81.1% (30/37) of pediatric ID specialists and 72.2% (52/72) of Neo responded to the survey. Some of the respondents (33.3% of ID and 59.6% of Neo) performed blood culture as a regular test irrespective of the indication. Approximately 40% of ID and 65.4% of Neo ordered only one set of blood culture in patients suspected with bacteremia. The most commonly used disinfectant for skin preparation was povidone-iodine, while the skin preparation method varied by institution. Approximately two-thirds of the institutions were monitoring the blood culture contamination rate, whereas relatively few provided staff with feedback on that rate. In addition, less than half of the institutions were providing regular staff training on blood culture (40% of ID and 28.8% of Neo).
CONCLUSIONS
The indication and methods of blood culture for children varied according to institution, and few hospitals exert effort in improving the quality of blood culture. Institutions have to strive constantly toward improvement of blood culture quality and evidence-based recommendations for pediatric blood cultures should be standardized.
MeSH Terms
Figure
Reference
-
References
1. Alahmadi YM, Aldeyab MA, McElnay JC, Scott MG, Darwish Elhajji FW, Magee FA, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect. 2011; 77:233–6.
Article2. Segal GS, Chamberlain JM. Resource utilization and contaminated blood cultures in children at risk for occult bacteremia. Arch Pediatr Adolesc Med. 2000; 154:469–73.
Article3. Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009; 47:1021–4.
Article4. Wilson ML. Clinical and Laboratory Standards Institute. M47A Principles and procedures for blood cultures: approved guideline. 1st Ed.Wayne: Clinical and Laboratory Standards Institute;2007.5. Shin JH, Song SA, Kim M, Kim S. Nationwide survey of blood culture performance regarding skin disinfection, blood collection and laboratory procedures. Korean J Clin Microbiol. 2011; 14:91–6.
Article6. Snyder SR, Favoretto AM, Baetz RA, Derzon JH, Madison BM, Mass D, et al. Effectiveness of practices to reduce blood culture contamination: a Laboratory Medicine Best Practices systematic review and metaanalysis. Clin Biochem. 2012; 45:999–1011.
Article7. Weinbaum FI, Lavie S, Danek M, Sixsmith D, Heinrich GF, Mills SS. Doing it right the first time: quality improvement and the contaminant blood culture. J Clin Microbiol. 1997; 35:563–5.
Article8. Kim NH, Kim M, Lee S, Yun NR, Kim KH, Park SW, et al. Effect of routine sterile gloving on contamination rates in blood culture: a cluster randomized trial. Ann Intern Med. 2011; 154:145–51.9. Clinical and Laboratory Standards Institute. GP41-A6 Procedures for the collection of diagnostic blood specimens by venipuncture; approved standard. 6th Ed.Wayne: Clinical and Laboratory Standards Institute;2007.10. Trautner BW, Clarridge JE, Darouiche RO. Skin antisepsis kits containing alcohol and chlorhexidine gluconate or tincture of iodine are associated with low rates of blood culture contamination. Infect Control Hosp Epidemiol. 2002; 23:397–401.
Article11. Isaacman DJ, Karasic RB, Reynolds EA, Kost SI. Effect of number of blood cultures and volume of blood on detection of bacteremia in children. J Pediatr. 1996; 128:190–5.
Article12. Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB Jr, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013; 57:e22–121.
Article13. Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996; 129:275–8.
Article14. Buttery JP. Blood cultures in newborns and children: optimising an everyday test. Arch Dis Child Fetal Neonatal Ed. 2002; 87:F25–8.
Article15. Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children's hospital. Pediatrics. 2007; 119:891–6.
Article16. Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014; 27:21–47.
Article17. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006; 19:788–802.
Article18. Park WB, Myung SJ, Oh MD, Lee J, Kim NJ, Kim EC, et al. Educational intervention as an effective step for reducing blood culture contamination: a prospective cohort study. J Hosp Infect. 2015; 91:111–6.
Article