Korean J Physiol Pharmacol.
2018 Nov;22(6):705-712. 10.4196/kjpp.2018.22.6.705.
Advanced tube formation assay using human endothelial colony forming cells for in vitro evaluation of angiogenesis
- Affiliations
-
- 1College of Pharmacy, Duksung Innovative Drug Center, Duksung Women's University, Seoul 01369, Korea. ktkang@duksung.ac.kr
- KMID: 2430093
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.705
Abstract
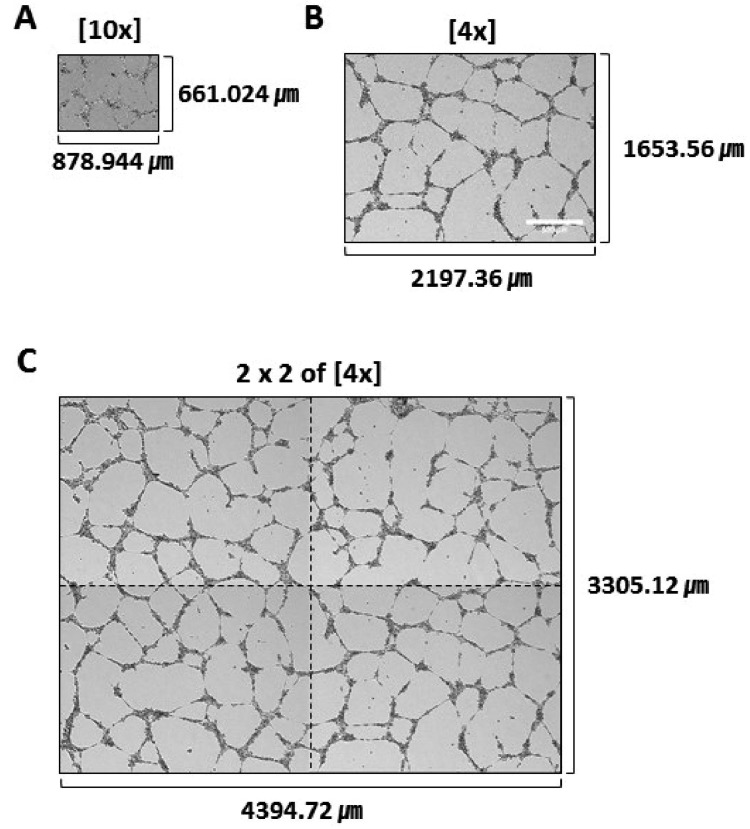
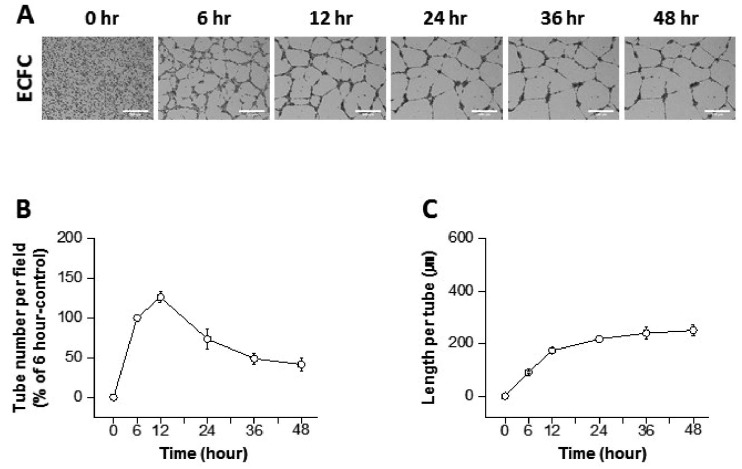
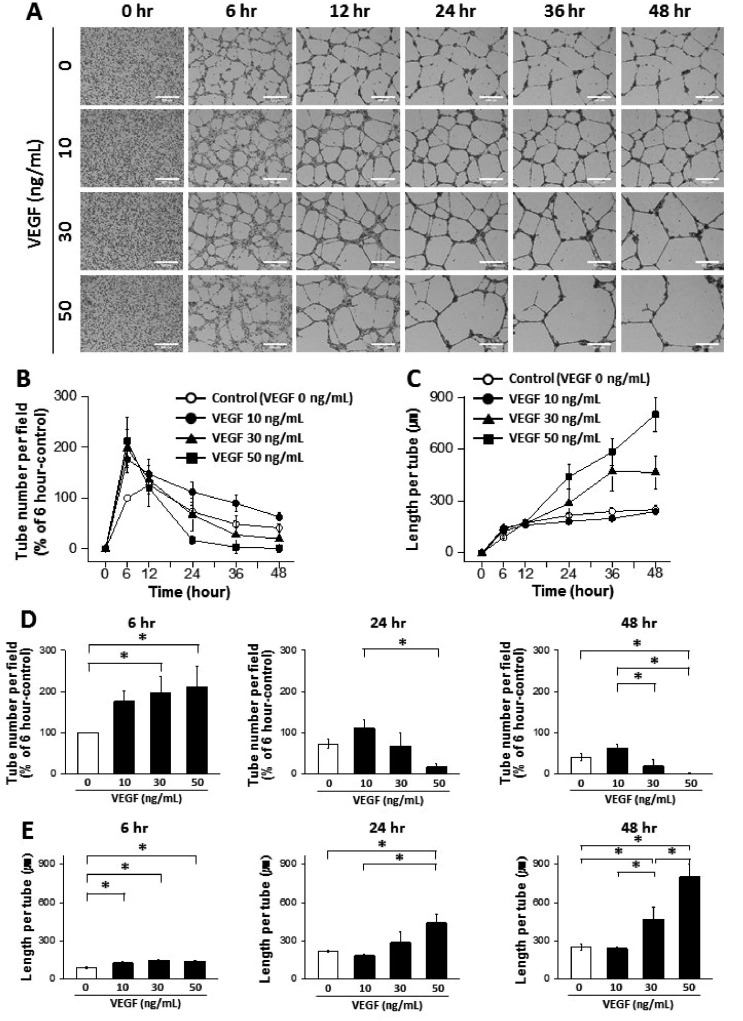
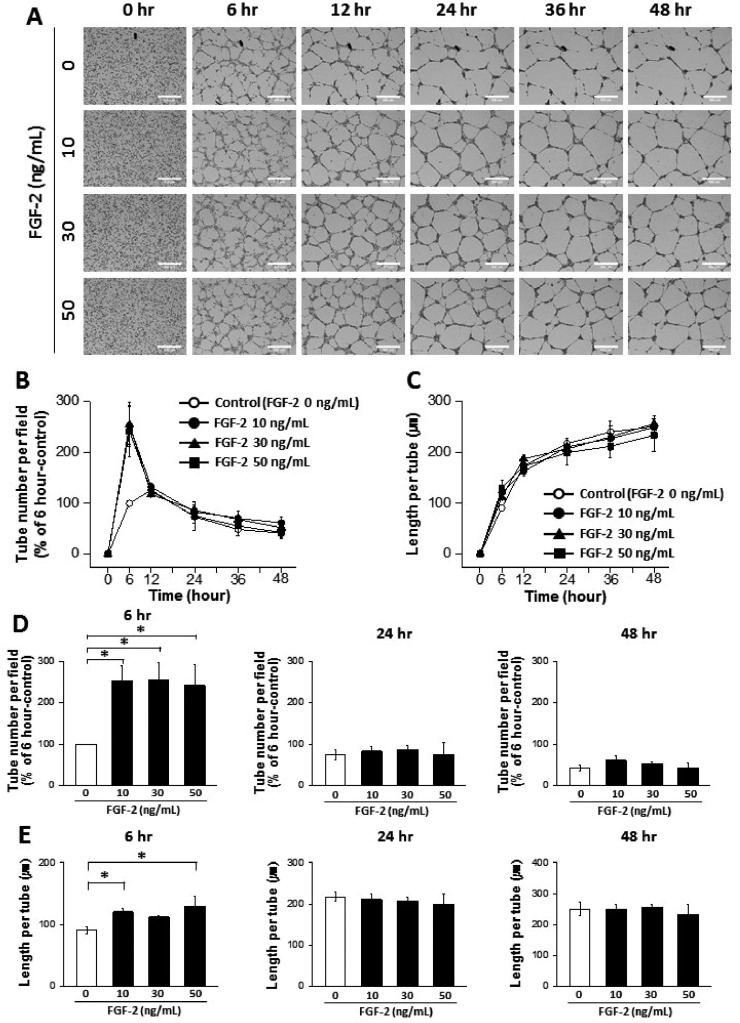
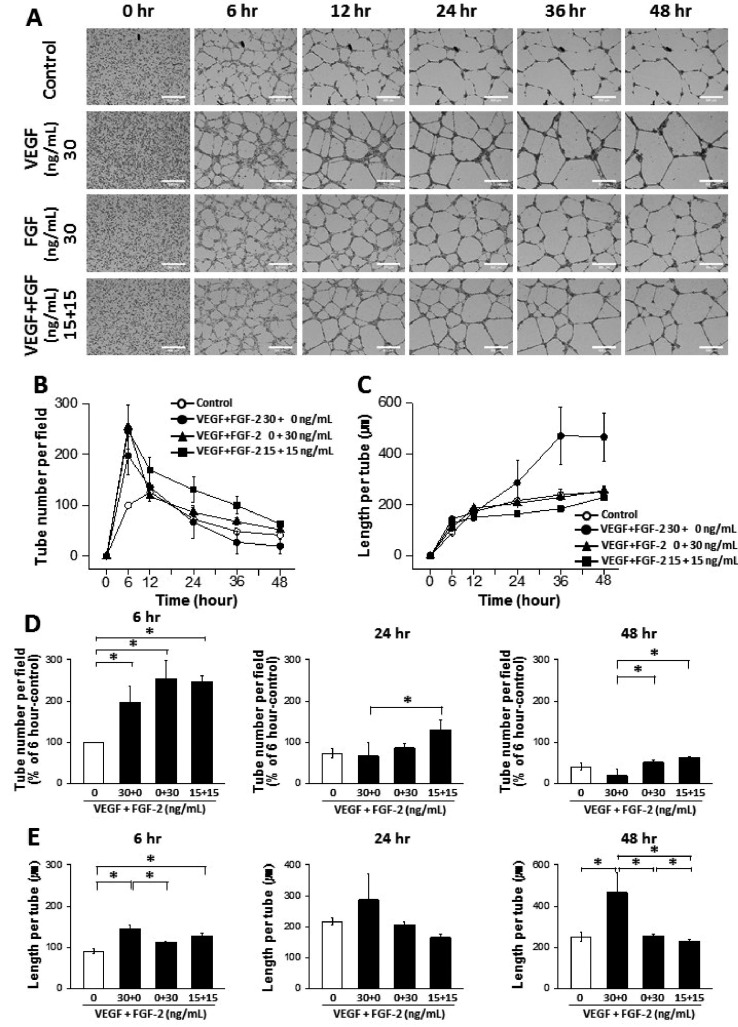
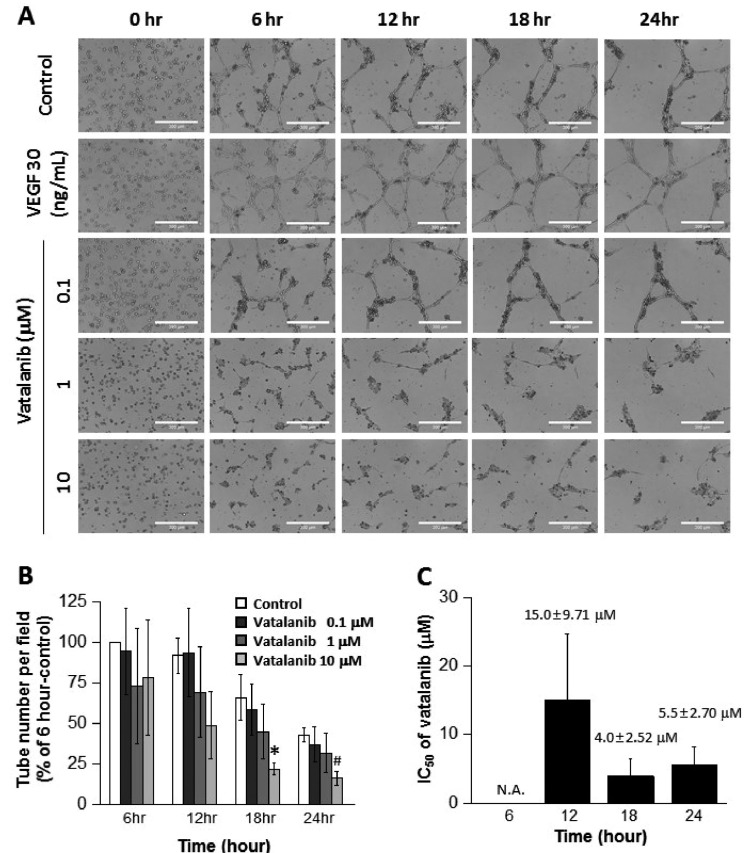
- The tube formation assay is a widely used in vitro experiment model to evaluate angiogenic properties by measuring the formation of tubular structures from vascular endothelial cells (ECs). in vitro experimental results are crucial when considered the advisability of moving forward to in vivo studies. Thus, the additional attentions to the in vitro assay is necessary to improve the quality of the pre-clinical data, leading to better decision-making for successful drug discovery. In this study, we improved the tube formation assay system in three aspects. First, we used human endothelial colony forming cells (ECFCs), which are endothelial precursors that have a robust proliferative capacity and more defined angiogenic characteristics compared to mature ECs. Second, we utilized a real-time cell recorder to track the progression of tube formation for 48 hours. Third, to minimize analysis error due to the limited observation area, we used image-stitching software to increase the microscope field of view to a 2×2 stitched area from the 4× object lens. Our advanced tube formation assay system successfully demonstrated the time-dependent dynamic progression of tube formation in the presence and absence of VEGF and FGF-2. Vatalanib, VEGF inhibitor, was tested by our assay system. Of note, IC₅₀ values of vatalanib was different at each observation time point. Collectively, these results indicate that our advanced tube formation assay system replicates the dynamic progression of tube formation in response to angiogenic modulators. Therefore, this new system provides a sensitive and versatile assay model for evaluating pro- or anti-angiogenic drugs.
Keyword
MeSH Terms
Figure
Reference
-
1. Gumkowski F, Kaminska G, Kaminski M, Morrissey LW, Auerbach R. Heterogeneity of mouse vascular endothelium. In vitro studies of lymphatic, large blood vessel and microvascular endothelial cells. Blood Vessels. 1987; 24:11–23. PMID: 3032311.2. Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010; 5:628–635. PMID: 20224563.
Article3. Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007; 109:4761–4768. PMID: 17327403.
Article4. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275:964–967. PMID: 9020076.
Article5. Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012; 110:624–637. PMID: 22343557.
Article6. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004; 104:2752–2760. PMID: 15226175.
Article7. Murasawa S, Asahara T. Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda). 2005; 20:36–42. PMID: 15653838.
Article8. Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002; 2:826–835. PMID: 12415253.
Article9. Iruela-Arispe ML, Dvorak HF. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost. 1997; 78:672–677. PMID: 9198237.
Article10. Melero-Martin JM, Bischoff J. Chapter 13. An in vivo experimental model for postnatal vasculogenesis. Methods Enzymol. 2008; 445:303–329. PMID: 19022065.11. Kim MK, Park HJ, Kim SR, Choi YK, Bae SK, Bae MK. Involvement of heme oxygenase-1 in orexin-A-induced angiogenesis in vascular endothelial cells. Korean J Physiol Pharmacol. 2015; 19:327–334. PMID: 26170736.
Article12. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003; 9:669–676. PMID: 12778165.
Article13. Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001; 22:201–207. PMID: 11282421.
Article14. Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O'Reilly T, Persohn E, Rösel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000; 60:2178–2189. PMID: 10786682.15. Bikfalvi A, Bicknell R. Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends Pharmacol Sci. 2002; 23:576–582. PMID: 12457776.
Article16. Kim DY, Jung SY, Kim YJ, Kang S, Park JH, Ji ST, Jang WB, Lamichane S, Lamichane BD, Chae YC, Lee D, Chung JS, Kwon SM. Hypoxia-dependent mitochondrial fission regulates endothelial progenitor cell migration, invasion, and tube formation. Korean J Physiol Pharmacol. 2018; 22:203–213. PMID: 29520173.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two-Cell Spheroid Angiogenesis Assay System Using Both Endothelial Colony Forming Cells and Mesenchymal Stem Cells
- Inhibition of Angiogenesis by the First Type I Repeat Peptides of Thrombospondin-1
- Effect of Triamcinolone on Angiogenesis-related Factors of Cultured Retinal Pigment Epithelial Cells
- Differential Angiogenic Responses of Human Endothelial Colony-Forming Cells to Different Molecular Subtypes of Breast Cancer Cells
- Expression of alpha3beta1 Integrin in ECV304 Endothelial Cells and Angiogenesis