Korean J Radiol.
2017 ;18(4):729-738. 10.3348/kjr.2017.18.4.729.
Value of Repeat Brain MRI in Children with Focal Epilepsy and Negative Findings on Initial MRI
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea. jhkate@skku.edu
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea.
- KMID: 2427242
- DOI: http://doi.org/10.3348/kjr.2017.18.4.729
Abstract
OBJECTIVE
To evaluate the value of repeat brain magnetic resonance imaging (MRI) in identifying potential epileptogenic lesions in children with initial MRI-negative focal epilepsy.
MATERIALS AND METHODS
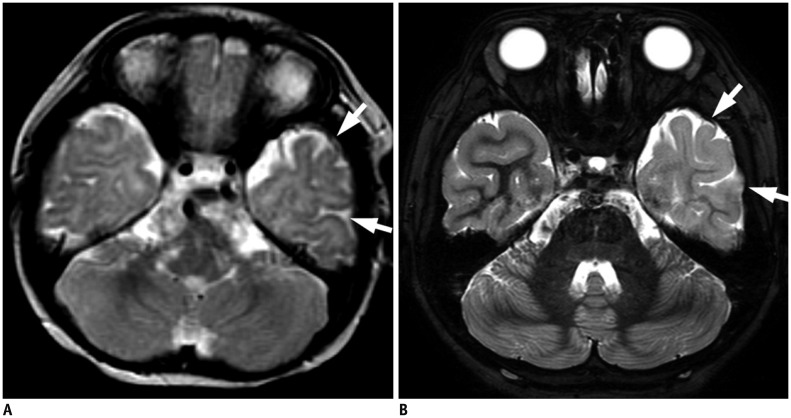
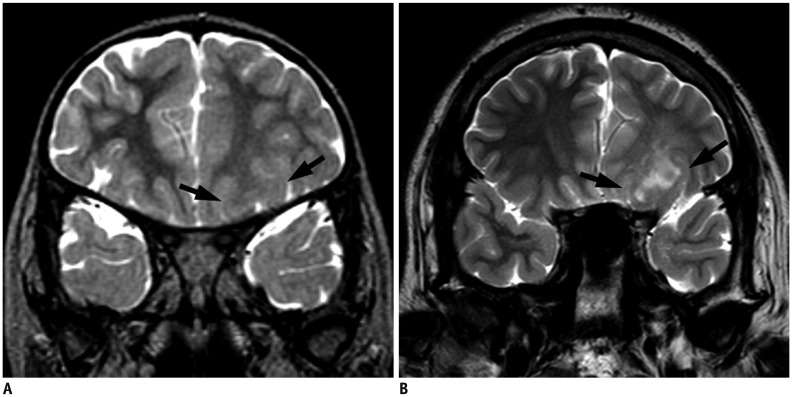
Our Institutional Review Board approved this retrospective study and waived the requirement for informed consent. During a 15-year period, 257 children (148 boys and 109 girls) with initial MRI-negative focal epilepsy were included. After re-evaluating both initial and repeat MRIs, positive results at repeat MRI were classified into potential epileptogenic lesions (malformation of cortical development and hippocampal sclerosis) and other abnormalities. Contributing factors for improved lesion conspicuity of the initially overlooked potential epileptogenic lesions were analyzed and classified into lesion factors and imaging factors.
RESULTS
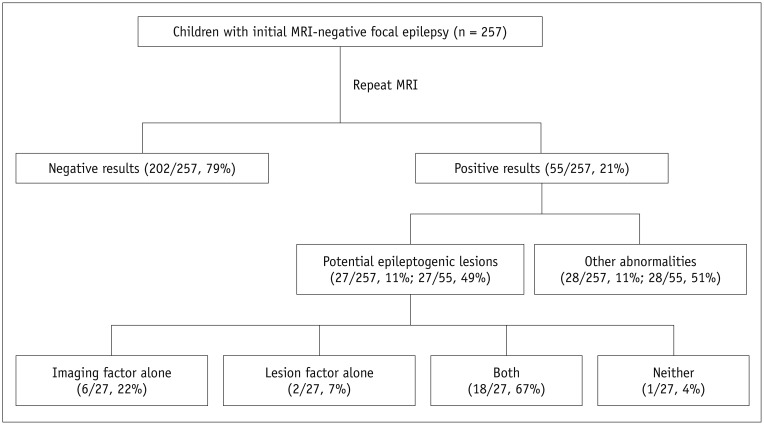
Repeat MRI was positive in 21% (55/257) and negative in 79% cases (202/257). Of the positive results, potential epileptogenic lesions comprised 49% (27/55) and other abnormalities comprised 11% of the cases (28/257). Potential epileptogenic lesions included focal cortical dysplasia (n = 11), hippocampal sclerosis (n = 10), polymicrogyria (n = 2), heterotopic gray matter (n = 2), microlissencephaly (n = 1), and cortical tumor (n = 1). Of these, seven patients underwent surgical resection. Contributing factors for new diagnoses were classified as imaging factors alone (n = 6), lesion factors alone (n = 2), both (n = 18), and neither (n = 1).
CONCLUSION
Repeat MRI revealed positive results in 21% of the children with initial MRI-negative focal epilepsy, with 50% of the positive results considered as potential epileptogenic lesions. Enhanced MRI techniques or considering the chronological changes of lesions on MRI may improve the diagnostic yield for identification of potential epileptogenic lesions on repeat MRI.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The Imaging of Localization Related Symptomatic Epilepsies: The Value of Arterial Spin Labelling Based Magnetic Resonance Perfusion
Chinmay Nagesh, Savith Kumar, Ramshekhar Menon, Bejoy Thomas, Ashalatha Radhakrishnan, Chandrasekharan Kesavadas
Korean J Radiol. 2018;19(5):965-977. doi: 10.3348/kjr.2018.19.5.965.Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.
Reference
-
1. Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999; 40:445–452. PMID: 10219270.
Article2. Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol. 2009; 66:1491–1499. PMID: 20008653.
Article3. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001; 345:311–318. PMID: 11484687.
Article4. French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993; 34:774–780. PMID: 8250525.
Article5. Gaillard WD, Chiron C, Cross JH, Harvey AS, Kuzniecky R, Hertz-Pannier L, et al. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009; 50:2147–2153. PMID: 19389145.
Article6. Berg AT, Testa FM, Levy SR, Shinnar S. Neuroimaging in children with newly diagnosed epilepsy: a community-based study. Pediatrics. 2000; 106:527–532. PMID: 10969098.7. See SJ, Jehi LE, Vadera S, Bulacio J, Najm I, Bingaman W. Surgical outcomes in patients with extratemporal epilepsy and subtle or normal magnetic resonance imaging findings. Neurosurgery. 2013; 73:68–76. discussion 76-77. PMID: 23615090.
Article8. Capraz IY, Kurt G, Akdemir Ö, Hirfanoglu T, Oner Y, Sengezer T, et al. Surgical outcome in patients with MRI-negative, PET-positive temporal lobe epilepsy. Seizure. 2015; 29:63–68. PMID: 26076845.
Article9. Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010; 89:310–318. PMID: 20227852.
Article10. Winston GP, Micallef C, Kendell BE, Bartlett PA, Williams EJ, Burdett JL, et al. The value of repeat neuroimaging for epilepsy at a tertiary referral centre: 16 years of experience. Epilepsy Res. 2013; 105:349–355. PMID: 23538269.
Article11. Nguyen DK, Rochette E, Leroux JM, Beaudoin G, Cossette P, Lassonde M, et al. Value of 3.0 T MR imaging in refractory partial epilepsy and negative 1.5 T MRI. Seizure. 2010; 19:475–478. PMID: 20673641.12. Knake S, Triantafyllou C, Wald LL, Wiggins G, Kirk GP, Larsson PG, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology. 2005; 65:1026–1031. PMID: 16217054.
Article13. Yoshida F, Morioka T, Hashiguchi K, Miyagi Y, Nagata S, Yamaguchi Y, et al. Appearance of focal cortical dysplasia on serial MRI after maturation of myelination. Childs Nerv Syst. 2008; 24:269–273. PMID: 17680248.
Article14. Yagishita A, Arai N, Maehara T, Shimizu H, Tokumaru AM, Oda M. Focal cortical dysplasia: appearance on MR images. Radiology. 1997; 203:553–559. PMID: 9114120.
Article15. Takanashi J, Barkovich AJ. The changing MR imaging appearance of polymicrogyria: a consequence of myelination. AJNR Am J Neuroradiol. 2003; 24:788–793. PMID: 12748072.16. Sankar R, Curran JG, Kevill JW, Rintahaka PJ, Shewmon DA, Vinters HV. Microscopic cortical dysplasia in infantile spasms: evolution of white matter abnormalities. AJNR Am J Neuroradiol. 1995; 16:1265–1272. PMID: 7677022.17. Eltze CM, Chong WK, Bhate S, Harding B, Neville BG, Cross JH. Taylor-type focal cortical dysplasia in infants: some MRI lesions almost disappear with maturation of myelination. Epilepsia. 2005; 46:1988–1992. PMID: 16393166.
Article18. Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010; 51:676–685. PMID: 20196795.
Article19. Vattipally VR, Bronen RA. MR imaging of epilepsy: strategies for successful interpretation. Neuroimaging Clin N Am. 2004; 14:349–372. PMID: 15324853.
Article20. Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. Classification system for malformations of cortical development: update 2001. Neurology. 2001; 57:2168–2178. PMID: 11785496.
Article21. Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GC, Bladin PF. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990; 40:1869–1875. PMID: 2247236.
Article22. Wald LL, Carvajal L, Moyher SE, Nelson SJ, Grant PE, Barkovich AJ, et al. Phased array detectors and an automated intensity-correction algorithm for high-resolution MR imaging of the human brain. Magn Reson Med. 1995; 34:433–439. PMID: 7500883.
Article23. McBride MC, Bronstein KS, Bennett B, Erba G, Pilcher W, Berg MJ. Failure of standard magnetic resonance imaging in patients with refractory temporal lobe epilepsy. Arch Neurol. 1998; 55:346–348. PMID: 9520008.
Article24. Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernández G, et al. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry. 2002; 73:643–647. PMID: 12438463.
Article25. Barkovich AJ. Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol. 2000; 21:1099–1109. PMID: 10871022.26. Davies KG, Hermann BP, Dohan FC Jr, Foley KT, Bush AJ, Wyler AR. Relationship of hippocampal sclerosis to duration and age of onset of epilepsy, and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res. 1996; 24:119–126. PMID: 8796360.
Article27. Tasch E, Cendes F, Li LM, Dubeau F, Andermann F, Arnold DL. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol. 1999; 45:568–576. PMID: 10319878.
Article28. Kadom N, Tsuchida T, Gaillard WD. Hippocampal sclerosis in children younger than 2 years. Pediatr Radiol. 2011; 41:1239–1245. PMID: 21735179.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between Patterns of Focal Epileptiform Discharges and Brain Lesions in Children with Focal Epilepsy
- The Correlation between ADHD and Brain MRI Findings in Children with Epilepsy
- Brain MRI findings of complex partial seizure in children
- Comparative Study for the Diagnostic Usefulness of Brain MRI and CT in Children with Seizure
- Significance of Polyspikes on Electroencephalography in Children with Focal Epilepsy