Korean J Radiol.
2017 ;18(4):710-721. 10.3348/kjr.2017.18.4.710.
Splenial Lesions of the Corpus Callosum: Disease Spectrum and MRI Findings
- Affiliations
-
- 1Department of Radiology, Gyeongsang National University School of Medicine, Jinju 52727, Korea. choids@gnu.ac.kr
- 2Gyeongsang Institute of Health Science, Gyeongsang National University School of Medicine, Jinju 52727, Korea.
- KMID: 2427240
- DOI: http://doi.org/10.3348/kjr.2017.18.4.710
Abstract
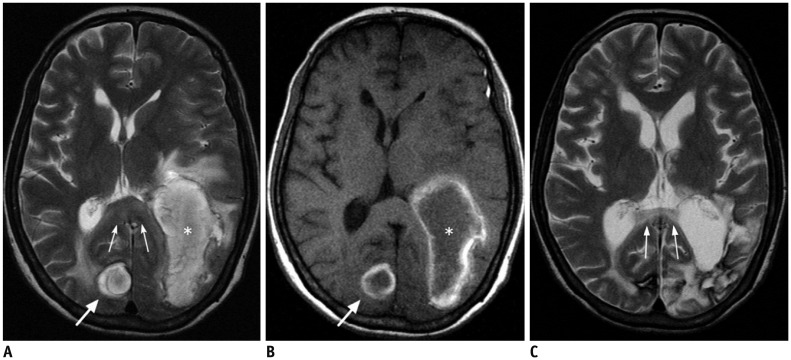
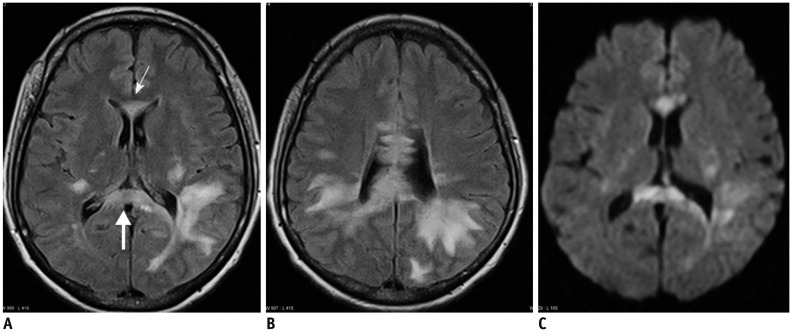
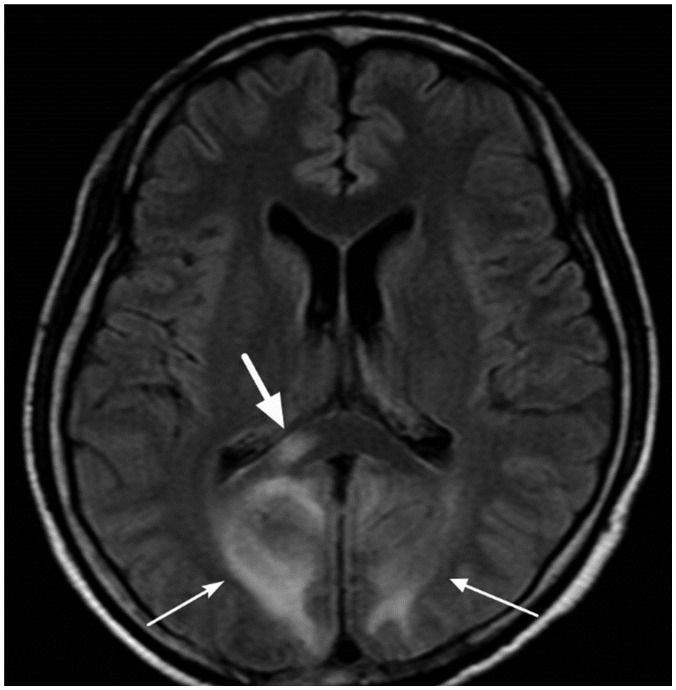
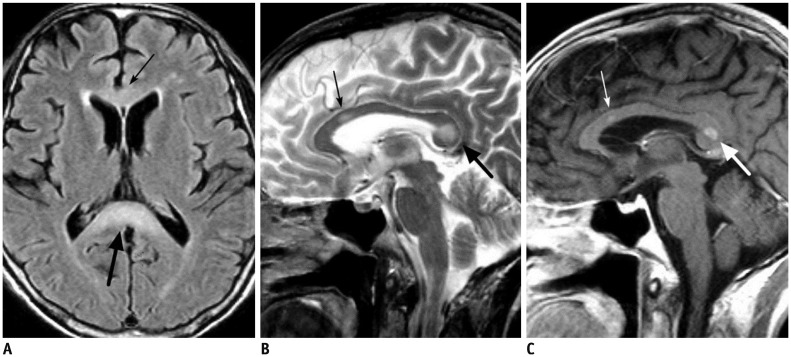
- The corpus callosum (CC) is the largest white matter structure in the brain, consisting of more than 200-250 million axons that provide a large connection mainly between homologous cerebral cortical areas in mirror image sites. The posterior end of the CC is the thickest part, which is called the splenium. Various diseases including congenital to acquired lesions including congenital anomalies, traumatic lesions, ischemic diseases, tumors, metabolic, toxic, degenerative, and demyelinating diseases, can involve the splenium of the CC and their clinical symptoms and signs are also variable. Therefore, knowledge of the disease entities and the imaging findings of lesions involving the splenium is valuable in clinical practice. MR imaging is useful for the detection and differential diagnosis of splenial lesions of the CC. In this study, we classify the disease entities and describe imaging findings of lesions involving the splenium of the CC based on our experiences and a review of the literature.
Keyword
MeSH Terms
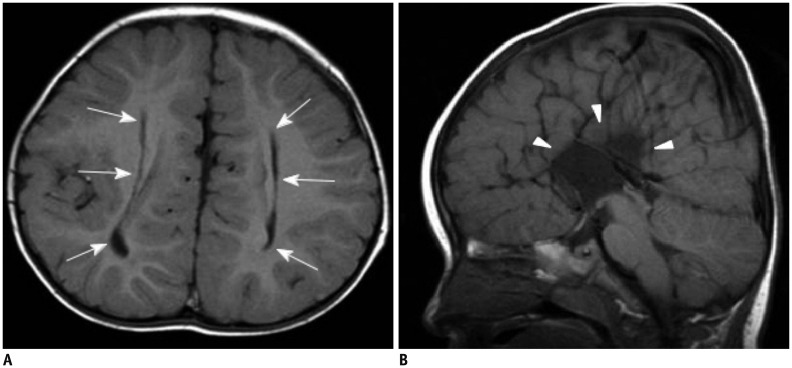
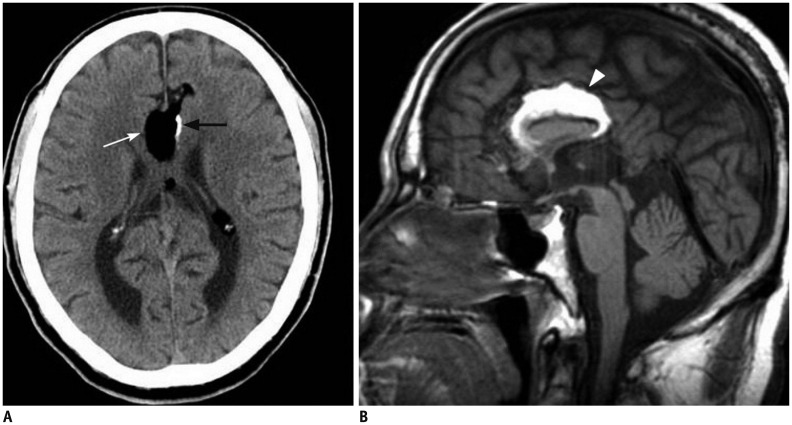
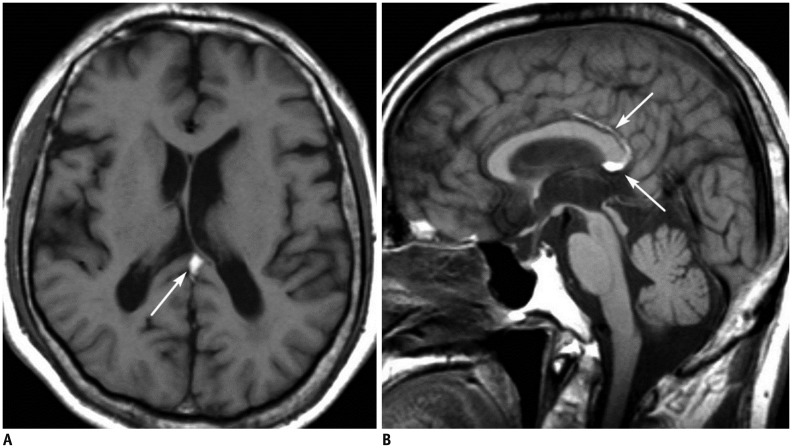
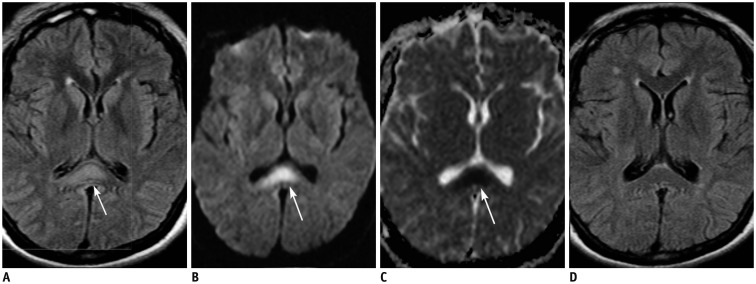
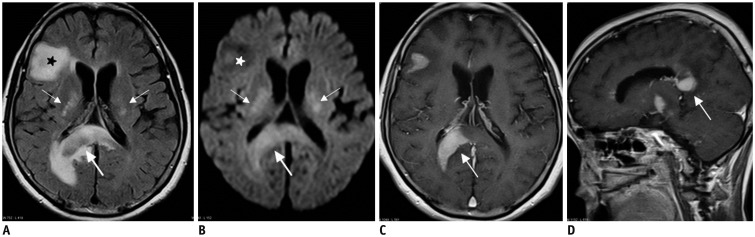
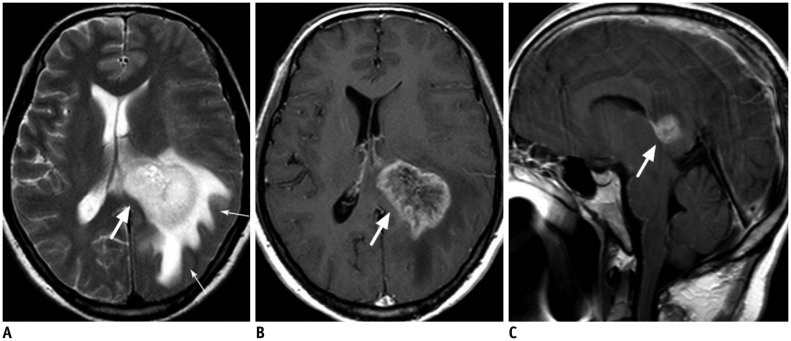
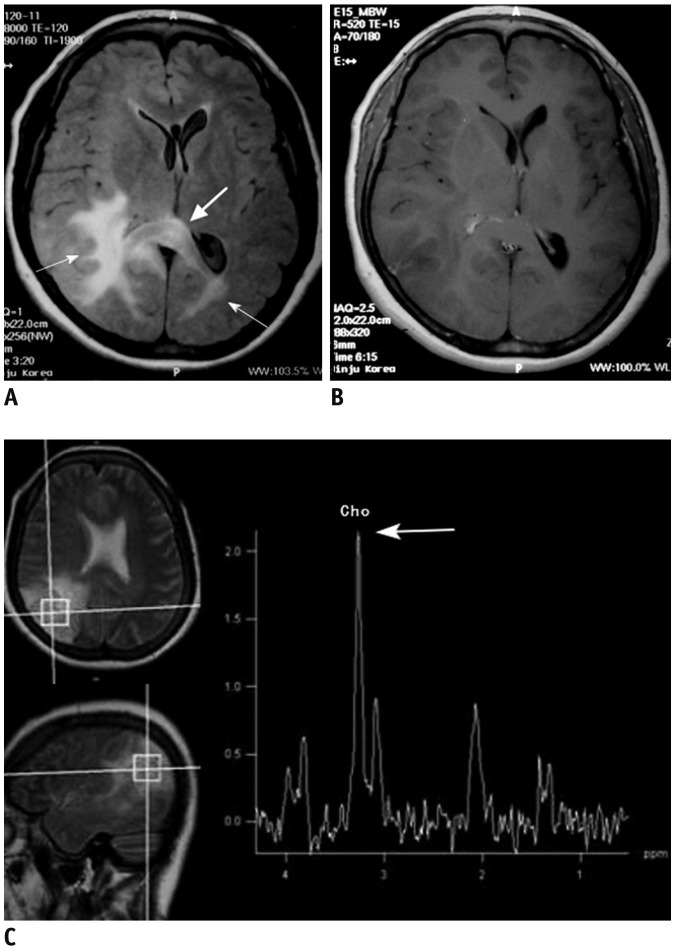
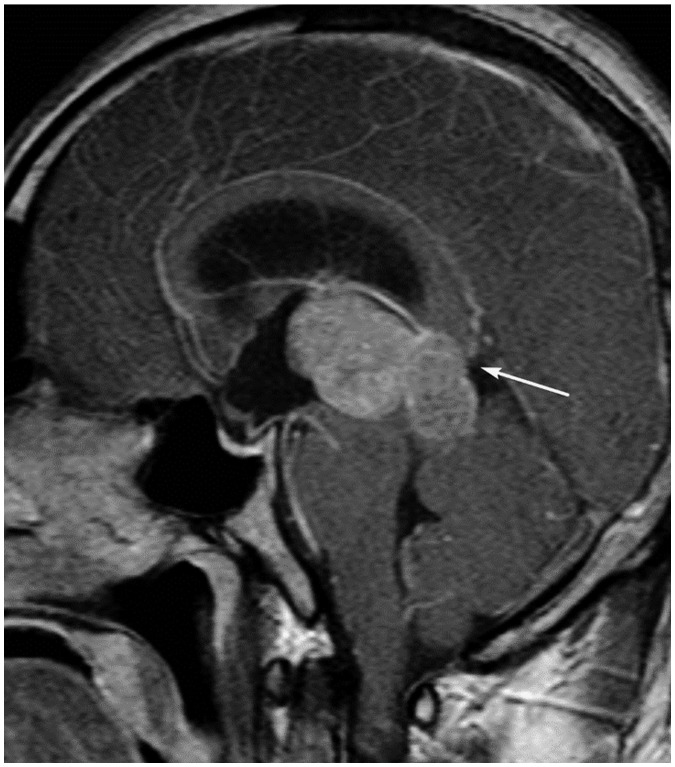
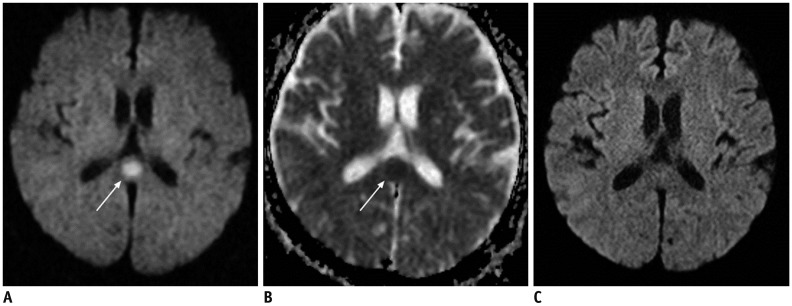
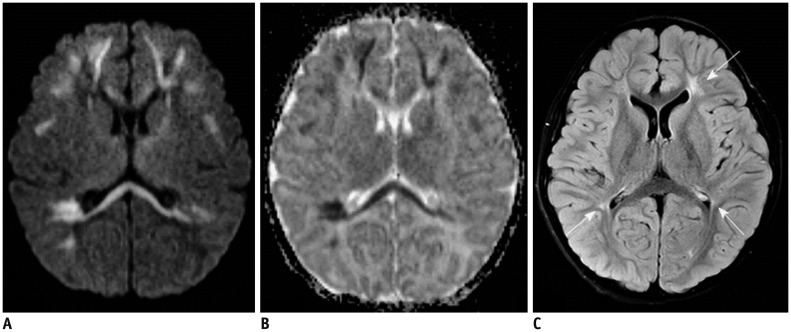
Figure
Reference
-
1. Lee SK, Kim DI, Kim J, Kim DJ, Kim HD, Kim DS, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics. 2005; 25:53–65. discussion 66-68. PMID: 15653586.
Article2. Doherty MJ, Jayadev S, Watson NF, Konchada RS, Hallam DK. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol. 2005; 62:433–437. PMID: 15767508.
Article3. Park MK, Hwang SH, Jung S, Hong SS, Kwon SB. Lesions in the splenium of the corpus callosum: clinical and radiological implications. Neurol Asia. 2014; 19:79–88.4. Li S, Sun X, Bai YM, Qin HM, Wu XM, Zhang X, et al. Infarction of the corpus callosum: a retrospective clinical investigation. PLoS One. 2015; 10:e0120409. PMID: 25785450.
Article5. Georgy BA, Hesselink JR, Jernigan TL. MR imaging of the corpus callosum. AJR Am J Roentgenol. 1993; 160:949–955. PMID: 8470609.
Article6. Barkovich AJ, Norman D. Anomalies of the corpus callosum: correlation with further anomalies of the brain. AJR Am J Roentgenol. 1988; 151:171–179. PMID: 3259802.
Article7. Truwit CL, Barkovich AJ. Pathogenesis of intracranial lipoma: an MR study in 42 patients. AJR Am J Roentgenol. 1990; 155:855–864. discussion 865. PMID: 2119122.
Article8. Ginat DT, Meyers SP. Intracranial lesions with high signal intensity on T1-weighted MR images: differential diagnosis. Radiographics. 2012; 32:499–516. PMID: 22411945.
Article9. Uchino A, Takase Y, Nomiyama K, Egashira R, Kudo S. Acquired lesions of the corpus callosum: MR imaging. Eur Radiol. 2006; 16:905–914. PMID: 16284771.
Article10. Provenzale JM. Imaging of traumatic brain injury: a review of the recent medical literature. AJR Am J Roentgenol. 2010; 194:16–19. PMID: 20028899.
Article11. Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002; 23:794–802. PMID: 12006280.12. Scheid R, Preul C, Gruber O, Wiggins C, von Cramon DY. Diffuse axonal injury associated with chronic traumatic brain injury: evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR Am J Neuroradiol. 2003; 24:1049–1056. PMID: 12812926.13. Chrysikopoulos H, Andreou J, Roussakis A, Pappas J. Infarction of the corpus callosum: computed tomography and magnetic resonance imaging. Eur J Radiol. 1997; 25:2–8. PMID: 9248790.
Article14. Kasow DL, Destian S, Braun C, Quintas JC, Kagetsu NJ, Johnson CE. Corpus callosum infarcts with atypical clinical and radiologic presentations. AJNR Am J Neuroradiol. 2000; 21:1876–1880. PMID: 11110540.15. Ho ML, Moonis G, Ginat DT, Eisenberg RL. Lesions of the corpus callosum. AJR Am J Roentgenol. 2013; 200:W1–W16. PMID: 23255767.
Article16. Chao CP, Zaleski CG, Patton AC. Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings. Radiographics. 2006; 26(Suppl 1):S159–S172. PMID: 17050513.
Article18. Kang EG, Jeon SJ, Choi SS, Song CJ, Yu IK. Diffusion MR imaging of hypoglycemic encephalopathy. AJNR Am J Neuroradiol. 2010; 31:559–564. PMID: 19875472.
Article19. Beltran-Marin M, Sadeghi N. Transient restricted diffusion in the splenium of the corpus callosum after brain surgery. JBR-BT. 2013; 96:92.
Article20. Lo CP, Chen SY, Lee KW, Chen WL, Chen CY, Hsueh CJ, et al. Brain injury after acute carbon monoxide poisoning: early and late complications. AJR Am J Roentgenol. 2007; 189:W205–W211. PMID: 17885032.
Article21. Beppu T. The role of MR imaging in assessment of brain damage from carbon monoxide poisoning: a review of the literature. AJNR Am J Neuroradiol. 2014; 35:625–631. PMID: 23598831.
Article22. Bourekas EC, Varakis K, Bruns D, Christoforidis GA, Baujan M, Slone HW, et al. Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am J Roentgenol. 2002; 179:251–257. PMID: 12076946.
Article23. Kim M, Kim HS. Emerging techniques in brain tumor imaging: what radiologists need to know. Korean J Radiol. 2016; 17:598–619. PMID: 27587949.
Article24. Gupta RK, Saksena S, Hasan KM, Agarwal A, Haris M, Pandey CM, et al. Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: serial diffusion tensor imaging. J Magn Reson Imaging. 2006; 24:549–555. PMID: 16888796.
Article25. Brass SD, Caramanos Z, Santos C, Dilenge ME, Lapierre Y, Rosenblatt B. Multiple sclerosis vs acute disseminated encephalomyelitis in childhood. Pediatr Neurol. 2003; 29:227–231. PMID: 14629906.
Article26. Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007; 28:1320–1327. PMID: 17698535.
Article27. Arbelaez A, Pajon A, Castillo M. Acute Marchiafava-Bignami disease: MR findings in two patients. AJNR Am J Neuroradiol. 2003; 24:1955–1957. PMID: 14625216.28. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007; 28:1652–1658. PMID: 17885234.
Article29. Hirotani M, Yabe I, Hamada S, Tsuji S, Kikuchi S, Sasaki H. Abnormal brain MRI signals in the splenium of the corpus callosum, basal ganglia and internal capsule in a suspected case with tuberculous meningitis. Intern Med. 2007; 46:505–509. PMID: 17443044.
Article30. Conti M, Salis A, Urigo C, Canalis L, Frau S, Canalis GC. Transient focal lesion in the splenium of the corpus callosum: MR imaging with an attempt to clinical-physiopathological explanation and review of the literature. Radiol Med. 2007; 112:921–935. PMID: 17885738.
Article31. Malhotra HS, Garg RK, Vidhate MR, Sharma PK. Boomerang sign: clinical significance of transient lesion in splenium of corpus callosum. Ann Indian Acad Neurol. 2012; 15:151–157. PMID: 22566735.
Article32. Yeom JS, Kim YS, Seo JH, Park JS, Park ES, Lim JY, et al. Distinctive pattern of white matter injury in neonates with rotavirus infection. Neurology. 2015; 84:21–27. PMID: 25471392.
Article33. Amarnath C, Helen Mary T, Periakarupan A, Gopinathan K, Philson J. Neonatal parechovirus leucoencephalitis-radiological pattern mimicking hypoxic-ischemic encephalopathy. Eur J Radiol. 2016; 85:428–434. PMID: 26781149.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transient Splenial Lesion of the Corpus Callosum in Patients with Infectious Disease
- A Case of Transient Isolated Splenial Lesion of the Corpus Callosum After New Onset Seizure
- Reversible Splenial Lesion in the Corpus Callosum on MRI after Ingestion of a Herbicide Containing Glufosinate Ammonium: A Case Report
- Transient splenial lesions of the corpus callosum and infectious diseases
- Agenesis of Corpus Callosum