Yonsei Med J.
2016 Nov;57(6):1446-1453. 10.3349/ymj.2016.57.6.1446.
The Relationship between Magnesium and Endothelial Function in End-Stage Renal Disease Patients on Hemodialysis
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Ewha Womans University, Seoul, Korea. kbchoi@ewha.ac.kr
- KMID: 2427163
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1446
Abstract
- PURPOSE
Chronic kidney disease (CKD) patients tend to have higher serum magnesium values than healthy population due to their positive balance of magnesium in kidney. Recent studies found that magnesium level is positively correlated with endothelial function. Therefore, this study was conducted to define the relationship between magnesium level and endothelial dysfunction in end stage renal disease (ESRD) patients on hemodialysis (HD).
MATERIALS AND METHODS
A total of 27 patients were included in this cross-sectional study. Iontophoresis with laser-Doppler flowmetry, flow mediated dilation (FMD), and carotid intima-media thickness were measured. Patients' average serum magnesium levels were measured over previous three months, including the examination month. Pearson's correlation coefficient analysis and multivariate regression model were used to define the association between magnesium and endothelial function.
RESULTS
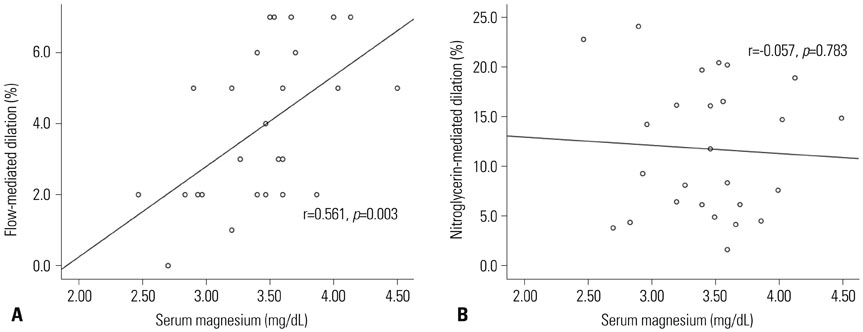
In the univariate analysis, higher magnesium levels were associated with better endothelium-dependent vasodilation (EDV) of the FMD in ESRD patients on HD (r=0.516, p=0.007). When the participants were divided into two groups according to the median magnesium level (3.47 mg/dL), there was a significant difference in EDV of FMD (less than 3.47 mg/dL, 2.8±1.7%; more than 3.47 mg/dL, 5.1±2.0%, p=0.004). In multivariate analysis, magnesium and albumin were identified as independent factors for FMD (β=1.794, p=0.030 for serum magnesium; β=3.642, p=0.012 for albumin).
CONCLUSION
This study demonstrated that higher serum magnesium level may be associated with better endothelial function in ESRD patients on HD. In the future, a large, prospective study is needed to elucidate optimal range of serum magnesium levels in ESRD on HD patients.
Keyword
MeSH Terms
-
Adult
Aged
Carotid Intima-Media Thickness
Cross-Sectional Studies
Endothelium, Vascular/pathology/physiology
Female
Humans
Kidney/*physiopathology
Kidney Failure, Chronic/*blood
Magnesium/*blood
Male
Middle Aged
Outcome Assessment (Health Care)
Prospective Studies
*Renal Dialysis
Renal Insufficiency, Chronic/blood
Vascular Diseases
Vasodilation
Magnesium
Figure
Reference
-
1. Altura BM, Altura BT. New perspectives on the role of magnesium in the pathophysiology of the cardiovascular system. I. Clinical aspects. Magnesium. 1985; 4:226–244.2. Reffelmann T, Ittermann T, Dörr M, Völzke H, Reinthaler M, Petersmann A, et al. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis. 2011; 219:280–284.
Article3. Amighi J, Sabeti S, Schlager O, Mlekusch W, Exner M, Lalouschek W, et al. Low serum magnesium predicts neurological events in patients with advanced atherosclerosis. Stroke. 2004; 35:22–27.
Article4. Ouchi Y, Tabata RE, Stergiopoulos K, Sato F, Hattori A, Orimo H. Effect of dietary magnesium on development of atherosclerosis in cholesterol-fed rabbits. Arteriosclerosis. 1990; 10:732–737.
Article5. Turgut F, Kanbay M, Metin MR, Uz E, Akcay A, Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008; 40:1075–1082.
Article6. McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation. 1991; 84:1273–1278.
Article7. Floege J, Johnson RJ, Feehally J. Comprehensive clinical nephrology. 4th ed. St. Louis: Elsevier Health Sciences;2010.8. Navarro-González JF, Mora-Fernández C, García-Pérez J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial. 2009; 22:37–44.
Article9. Truttmann AC, Faraone R, Von Vigier RO, Nuoffer JM, Pfister R, Bianchetti MG. Maintenance hemodialysis and circulating ionized magnesium. Nephron. 2002; 92:616–621.
Article10. Celermajer DS, Sorensen K, Ryalls M, Robinson J, Thomas O, Leonard JV, et al. Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol. 1993; 22:854–858.
Article11. Cupisti A, Rossi M, Placidi S, Caprioli R, Morelli E, Vagheggini G, et al. Responses of the skin microcirculation to acetylcholine and to sodium nitroprusside in chronic uremic patients. Int J Clin Lab Res. 2000; 30:157–162.
Article12. Niwayama J, Sanaka T. Development of a new method for monitoring blood purification: the blood flow analysis of the head and foot by laser Doppler blood flowmeter during hemodialysis. Hemodial Int. 2005; 9:56–62.
Article13. Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and sodium nitroprusside in patients with NIDDM. Diabetologia. 1995; 38:1337–1344.
Article14. Davis KR, Ponnampalam J, Hayman R, Baker PN, Arulkumaran S, Donnelly R. Microvascular vasodilator response to acetylcholine is increased in women with pre-eclampsia. BJOG. 2001; 108:610–614.
Article15. Yanase T, Nasu S, Mukuta Y, Shimizu Y, Nishihara T, Okabe T, et al. Evaluation of a new carotid intima-media thickness measurement by B-mode ultrasonography using an innovative measurement software, intimascope. Am J Hypertens. 2006; 19:1206–1212.
Article16. Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002; 105:546–549.
Article17. Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994; 24:1468–1474.
Article18. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992; 340:1111–1115.
Article19. Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation. 2000; 102:2739–2744.
Article20. Ma J, Folsom AR, Melnick SL, Eckfeldt JH, Sharrett AR, Nabulsi AA, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1995; 48:927–940.
Article21. Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1998; 136:480–490.
Article22. Wiles ME, Wagner TL, Weglicki WB. Effect of acute magnesium deficiency (MgD) on aortic endothelial cell (EC) oxidant production. Life Sci. 1997; 60:221–236.
Article23. Dickens BF, Weglicki WB, Li YS, Mak IT. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992; 311:187–191.
Article24. Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000; 102:2353–2358.
Article25. Mordes JP, Wacker WE. Excess magnesium. Pharmacol Rev. 1977; 29:273–300.26. Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013; 168:344–351.
Article27. Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006; 27:503–508.
Article28. Northcott CA, Watts SW. Low [Mg2+]e enhances arterial spontaneous tone via phosphatidylinositol 3-kinase in DOCA-salt hypertension. Hypertension. 2004; 43:125–129.
Article29. Ozkor MA, Quyyumi AA. Endothelium-derived hyperpolarizing factor and vascular function. Cardiol Res Pract. 2011; 2011:156146.
Article30. Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005; 563(Pt 3):965–973.
Article31. Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol. 2000; 129:184–192.
Article32. Noon JP, Walker BR, Hand MF, Webb DJ. Studies with iontophoretic administration of drugs to human dermal vessels in vivo: cholinergic vasodilatation is mediated by dilator prostanoids rather than nitric oxide. Br J Clin Pharmacol. 1998; 45:545–550.
Article33. Khan F, Davidson NC, Littleford RC, Litchfield SJ, Struthers AD, Belch JJ. Cutaneous vascular responses to acetylcholine are mediated by a prostanoid-dependent mechanism in man. Vasc Med. 1997; 2:82–86.
Article34. Durand S, Tartas M, Bouyé P, Koïtka A, Saumet JL, Abraham P. Prostaglandins participate in the late phase of the vascular response to acetylcholine iontophoresis in humans. J Physiol. 2004; 561(Pt 3):811–819.
Article35. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986; 74:1399–1406.
Article36. Tzanakis I, Virvidakis K, Tsomi A, Mantakas E, Girousis N, Karefyllakis N, et al. Intra- and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes Res. 2004; 17:102–108.37. Navarro JF, Macía ML, Gallego E, Méndez ML, Chahín J, García-Nieto V, et al. Serum magnesium concentration and PTH levels. Is long-term chronic hypermagnesemia a risk factor for adynamic bone disease? Scand J Urol Nephrol. 1997; 31:275–280.
Article38. Baradaran A, Nasri H. Correlation of serum magnesium with serum parathormone levels in patients on regular hemodialysis. Saudi J Kidney Dis Transpl. 2006; 17:344–350.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Studyof Dermal Mast Cells Number in End Stage of Renal Failure
- Changes in Flow-Mediated Vasodilation after a Single Session of Hemodialysis
- Effect of Hemodialysis on Taste Acuity in Patient with End-Stage Renal Disease
- Successful Treatment of Small-Cell Lung Cancer With Irinotecan in a Hemodialysis Patient With End-Stage Renal Disease
- Phenomenology on the Hemodialysis Experience of Patients with End-Stage Renal Disease