J Korean Ophthalmol Soc.
2010 May;51(5):693-699.
The Role of Electroretinography in Assessing the Progression of Diabetic Retinopathy
- Affiliations
-
- 1Department of Ophthalmology, Hallym University College of Medicine, Seoul, Korea.
- 2Department of Ophthalmology, Soonchunhyang University College of Medicine, Bucheon, Korea. yhohn@schbc.ac.kr
Abstract
- PURPOSE
To investigate the clinical significance of electroretinographic (ERG) responses, including the photopic negative response parameter, in assessing the progression of diabetic retinopathy.
METHODS
Standard flash ERG was tested on 28 normal controls and 143 patients who were diagnosed with diabetes mellitus. Of those, 97 patients had diabetic retinopathy in different stages. Electroretinography was performed according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standards. Amplitudes and implicit times of ERG responses, including the photopic negative response (PhNR), were compared at different stages of diabetic retinopathy.
RESULTS
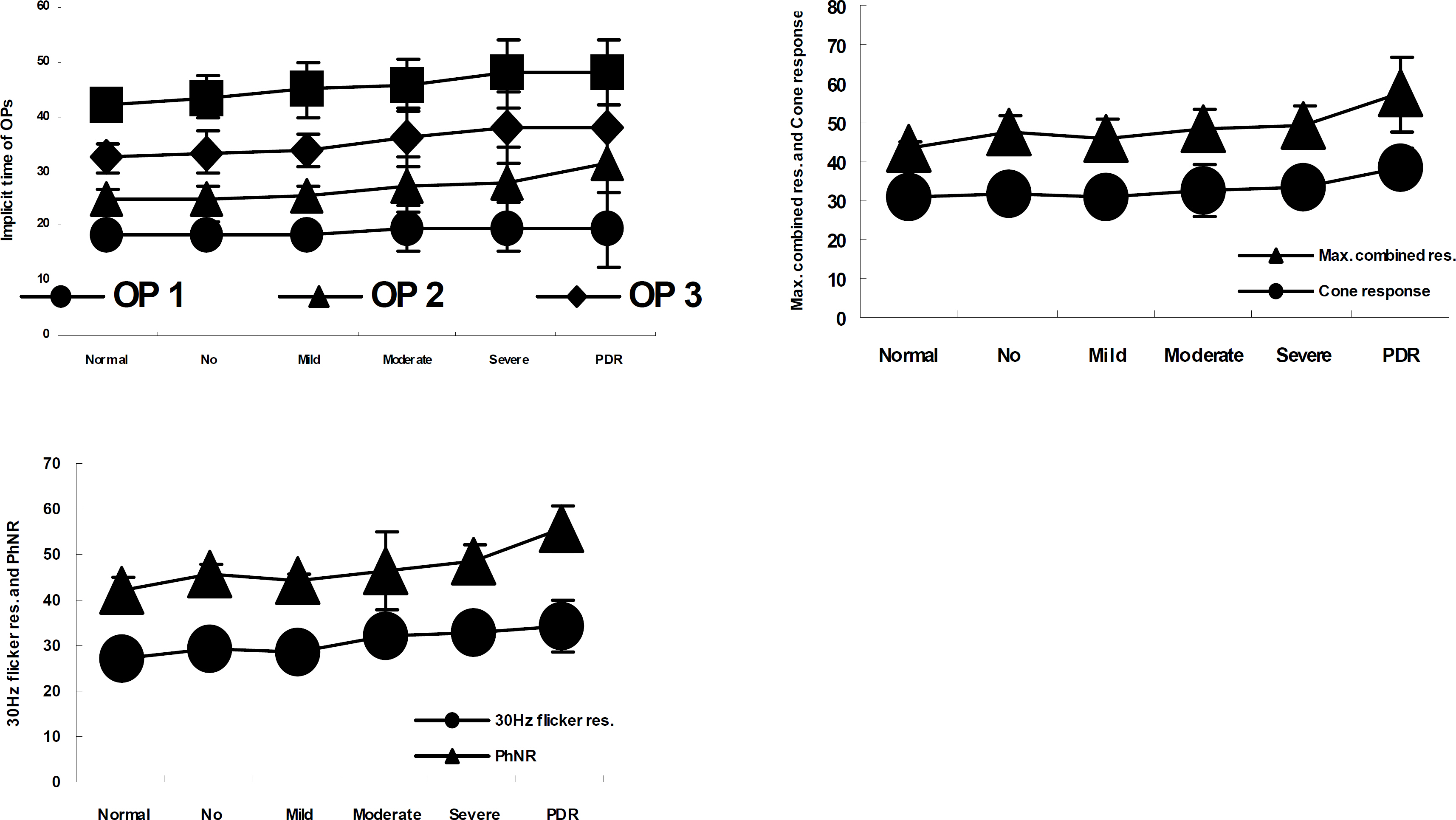
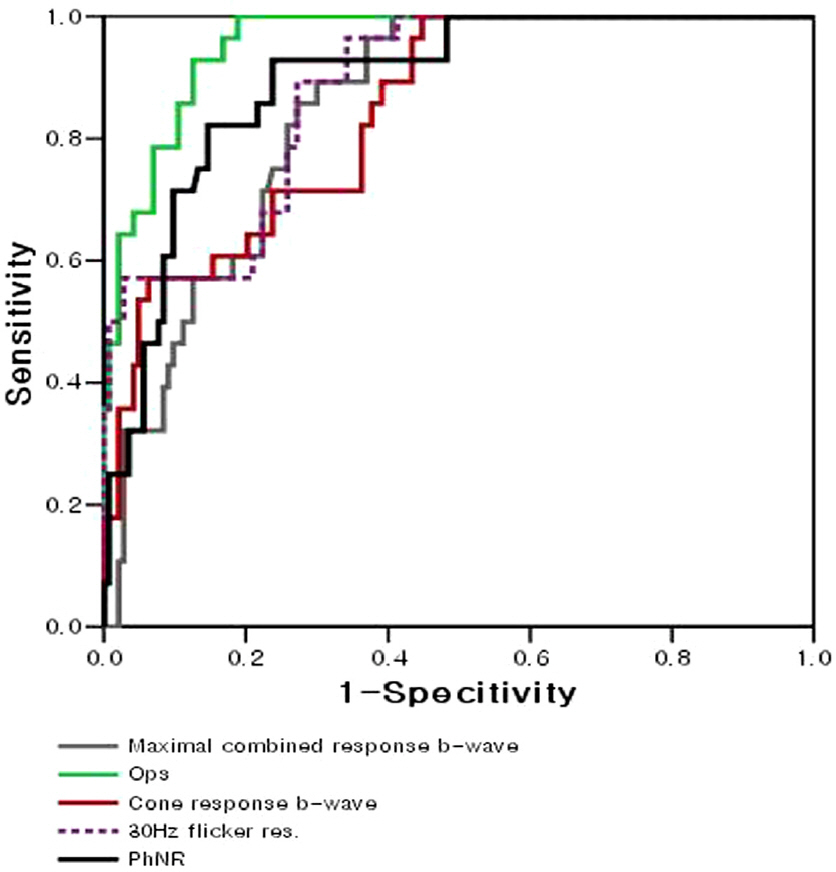
Amplitudes of oscillatory potentials were significantly reduced in mild NPDR. Cone b-wave amplitude, 30--Hz flicker response, and PhNR were significantly reduced in moderate NPDR.
CONCLUSIONS
These results suggest that oscillatory potentials are good indicators of retinal function change in the early stage of diabetic retinopathy, and the appropriate results of the cone b-wave, 30-Hz flicker response and PhNR tests are good indicators of moderate NPDR.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Wallow IH, Engerman RL. Permeability and patency of retinal blood vessels in experimental diabetes. Invest Ophthalmol Vis Sci. 1977; 16:447–61.2. Wallow IH, Geldner PS. Endothelial fenestrae in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1980; 19:1176–83.3. Han YK, Ohn YH. Changes of ERG parameters in diabetic retinopathy. J Korean Ophthalmol Soc. 2000; 41:149–55.4. Yonemura D, Kawasaki K. New approaches to ophthalmic electro-diagnosis by retinal oscillatory potentials, drug-induced responses from retinal pigment epithelium and cone potential. Doc Ophthalmol. 1974; 48:163–222.5. Bresnick GH, Palta M. Temporal aspects of the electroretinogram in diabetic retinopathy. Arch Ophthalmol. 1987; 105:660–4.
Article6. Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: electroretinographical and morphological observations. Exp Eye Res. 2002; 74:615–25.
Article7. Bresnick GH, Palta M. Oscillatory potential amplitudes: Relation to severity of diabetic retinopathy. Arch Ophthamol. 1987; 105:929–33.8. Ogden TE. Oscillatory waves of the primate electroretinogram. Vision Res. 1973; 13:1059–74.9. Vigh J, Solessio E, Morgans CW, Lasater EM. Ionic mechanisms medi-ating oscillatory membrane potentials in wide-field retinal amacrine cells. J Neurophysiol. 2003; 90:431–43.10. Hancock HA, Kraft TW. Oscillatory potential analysis and ERGs of normal and diabetic rats. Invest Ophthalmol Vis Sci. 2004; 45:1002–8.
Article11. Juen S, Kieselbach GF. Electrophysiological changes in juvenile abdominals without retinopathy. Arch Ophthalmol. 1990; 108:372–5.12. Vadalà M, Anastasi M, Lodato G, Cillino S. Electroretinographic abdominal potentials in insulin-dependent diabetes patients:a long-term follow-up. Acta Ophthalmol Scand. 2002; 80:305–9.13. Yoshida A, Kojima M, Ogasawara H, Ishiko S. Oscillatory potentials and permeability of the blood-retinal barrier in noninsulin-dependent diabetic patients without retinopathy. Ophthalmology. 1991; 98:1266–71.
Article14. Holopigian K, Seiple W, Lorenzo M, Carr R. A comparison of abdominal and scotopic electroretinographic changes in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1992; 33:2773–80.15. Seo DS, Lee SY, Park SK, Roh JH. Usefulness of standard abdominal in the early diagnosis of diabetic retinopathy: analysis abdominal Receiver Operating Characteristic (ROC) curve. J Korean Ophthalmol Soc. 1999; 40:1552–8.16. Kwak MS, Kim SH. The electroretinogram a- and b-wave in diabetic retinopathy. J Korean Ophthalmol Soc. 1994; 35:1081–7.17. Viswanathan S, Frishman LJ, Robson JG, et al. The photopic negative response of the macaque electroretinogram: reduction by abdominal glaucoma. Invest Ophthalmol Vis Sci. 1999; 40:1124–36.18. Viswanathan S, Frishman LJ, Robson JG, Walters JW. The photopic negative response of the flash electroretinogram in primary open abdominal glaucoma. Invest Ophthalmol Vis Sci. 2001; 42:514–22.19. Gotoh Y, Machida S, Tazawa Y. Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol. 2004; 122:341–6.20. Colotto A, Falsini B, Salgarello T, et al. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000; 41:2205–11.21. Ng YK, Zeng XX, Ling EA. Expression of glutamate receptors and calcium-binding proteins in the retina of streptozotocin-induced abdominal rats. Brain Res. 2004; 1018:66–72.22. Pulido JE, Pulido JS, Erie JC, et al. A role for excitatory amino acids in diabetic eye disease. Exp Diabetes Res. 2007; 2007:36150.
Article23. Santiago AR, Hughes JM, Kamphuis W, et al. Diabetes changes abdominal glutamate receptor subunit expression level in the human retina. Brain Res. 2008; 1198:153–9.24. Kizawa J, Machida S, Kobayashi T, et al. Changes of oscillatory abdominal and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol. 2006; 50:367–73.25. Kim HD, Park SH, Park SE, Ohn YH. Photopic negative response (PhNR) in normal subjects. J Korean Ophthalmol Soc. 2009; 50:1531–8.
Article26. Sieving PA, Frishman LJ, Steinberg RH. Scotopic threshold response of proximal retina in cat. J Neurophysiol. 1986; 56:1049–61.
Article27. Aylward GW. The scotopic threshold response in diabetic retinopathy. Eye. 1989; 3:626–37.
Article28. Attawia MA, Nayak RC. Circulating antipericyte autoantibodies in diabetic retinopathy. Retina. 1999; 19:390–400.
Article29. Kastelan S, Zjacić-Rotkvić V, Kastelan Z. Could diabetic retinopathy be an autoimmune disease? Medical Hypotheses. 2007; 68:1016–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of ERG Parameters in Diabetic Retinopathy
- The Influence of Axial Myopia on Diabetic Retinopathy
- The Influences of Arteriosclerosis on the Development and Progression of Diabetic Retinopathy
- Oscillatory potentials of local macular ERG in diabetic retinopathy
- Influence of Phacoemulsification on the Progression of Diabetic Retinopathy