Yonsei Med J.
2018 Nov;59(9):1041-1048. 10.3349/ymj.2018.59.9.1041.
Heat Shock Factor 1 Predicts Poor Prognosis of Gastric Cancer
- Affiliations
-
- 1Department of Biomedical Science, College of Natural Science, Chosun University, Gwangju, Korea. heaven1472@chosun.ac.kr
- 2Department of Pathology, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Biochemistry & Molecular Biology, Yonsei University College of Medicine, Seoul, Korea. khchun@yuhs.ac
- 4Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2422488
- DOI: http://doi.org/10.3349/ymj.2018.59.9.1041
Abstract
- PURPOSE
Heat shock factor 1 (HSF1) is a key regulator of the heat shock response and plays an important role in various cancers. However, the role of HSF1 in gastric cancer is still unknown. The present study evaluated the function of HSF1 and related mechanisms in gastric cancer.
MATERIALS AND METHODS
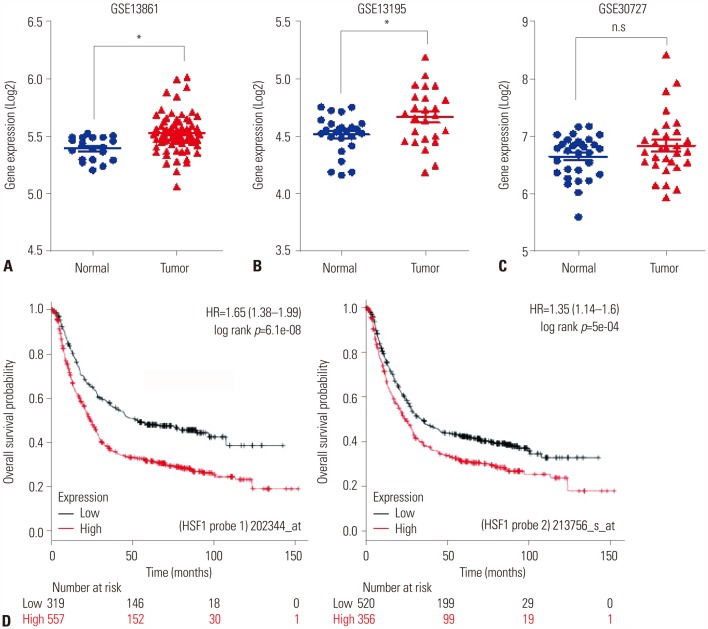
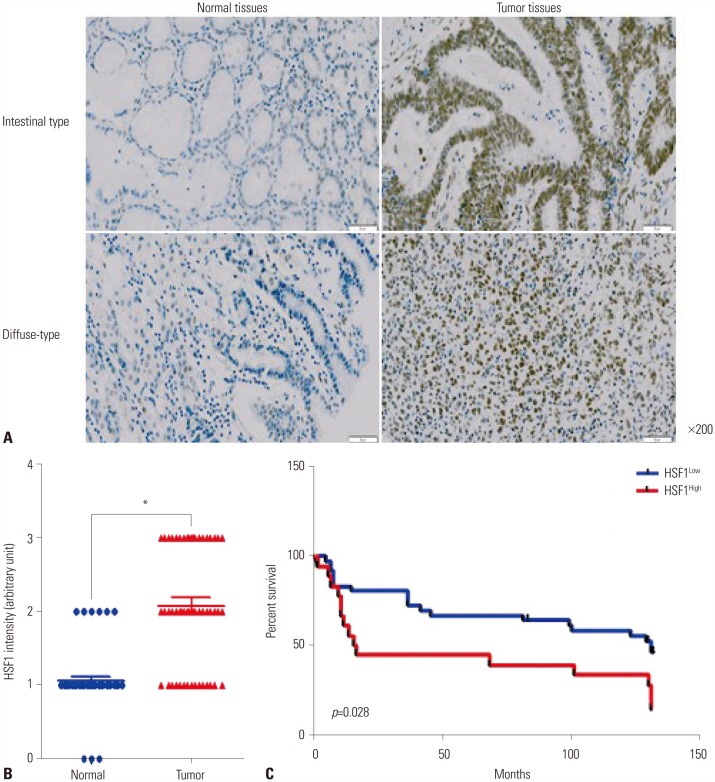
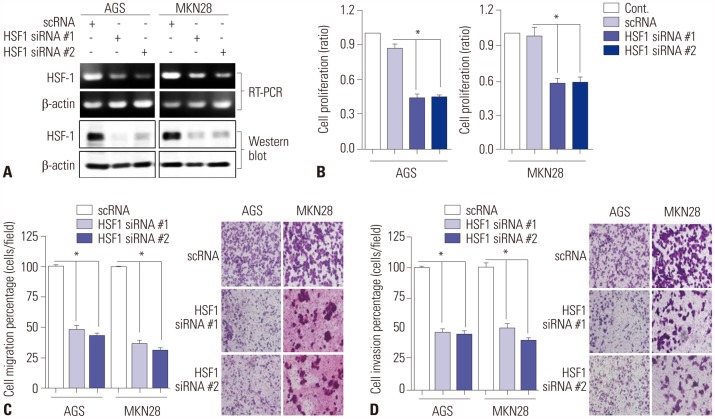
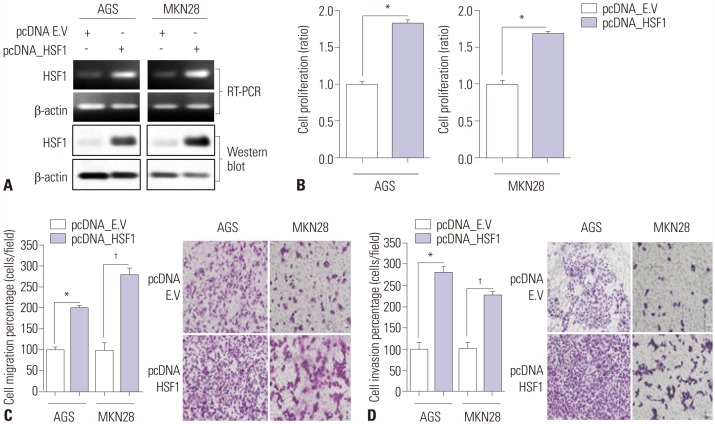
The expression levels of HSF1 in normal and gastric cancer tissues were compared using cDNA microarray data from the NCBI Gene Expression Omnibus (GEO) dataset. The proliferation of gastric cancer cells was analyzed using the WST assay. Transwell migration and invasion assays were used to evaluate the migration and invasion abilities of gastric cancer cells. Protein levels of HSF1 were analyzed using immunohistochemical staining of tissue microarrays from patients with gastric cancer.
RESULTS
HSF1 expression was significantly higher in gastric cancer tissue than in normal tissue. Knockdown of HSF1 reduced the proliferation, migration, and invasion of gastric cancer cells, while HSF1 overexpression promoted proliferation, migration, and invasion of gastric cancer cells. Furthermore, HSF1 promoted the proliferation of gastric cancer cells in vivo. In Kaplan-Meier analysis, high levels of HSF1 were associated with poor prognosis for patients with gastric cancer (p=0.028).
CONCLUSION
HSF1 may be closely associated with the proliferation and motility of gastric cancer cells and poor prognosis of patients with gastric cancer. Accordingly, HSF1 could serve as a prognostic marker for gastric cancer.
MeSH Terms
Figure
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID: 25651787.
Article2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–132. PMID: 26808342.
Article3. Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014; 20:4483–4490. PMID: 24782601.
Article4. Link A, Kupcinskas J. MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: current insights and future perspectives. World J Gastroenterol. 2018; 24:3313–3329. PMID: 30122873.
Article5. Ji X, Yan Y, Bu ZD, Li ZY, Wu AW, Zhang LH, et al. The optimal extent of gastrectomy for middle-third gastric cancer: distal subtotal gastrectomy is superior to total gastrectomy in short-term effect without sacrificing long-term survival. BMC Cancer. 2017; 17:345. PMID: 28526077.
Article6. Yuan DD, Zhu ZX, Zhang X, Liu J. Targeted therapy for gastric cancer: current status and future directions (Review). Oncol Rep. 2016; 35:1245–1254. PMID: 26718131.
Article7. Kanat O, O'Neil B, Shahda S. Targeted therapy for advanced gastric cancer: a review of current status and future prospects. World J Gastrointest Oncol. 2015; 7:401–410. PMID: 26690491.
Article8. Lin Y, Wu Z, Guo W, Li J. Gene mutations in gastric cancer: a review of recent next-generation sequencing studies. Tumour Biol. 2015; 36:7385–7394. PMID: 26364057.
Article9. Calderwood SK. HSF1, a versatile factor in tumorogenesis. Curr Mol Med. 2012; 12:1102–1107. PMID: 22804234.
Article10. Tomanek L, Somero GN. Interspecific- and acclimation-induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat-shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): implications for regulation of hsp gene expression. J Exp Biol. 2002; 205(Pt 5):677–685. PMID: 11907057.11. Neueder A, Gipson TA, Batterton S, Lazell HJ, Farshim PP, Paganetti P, et al. HSF1-dependent and -independent regulation of the mammalian in vivo heat shock response and its impairment in Huntington's disease mouse models. Sci Rep. 2017; 7:12556. PMID: 28970536.
Article12. Zhang Y, Huang L, Zhang J, Moskophidis D, Mivechi NF. Targeted disruption of hsf1 leads to lack of thermotolerance and defines tissue-specific regulation for stress-inducible Hsp molecular chaperones. J Cell Biochem. 2002; 86:376–393. PMID: 12112007.13. Gómez AV, Córdova G, Munita R, Parada GE, Barrios ÁP, Cancino GI, et al. Characterizing HSF1 binding and post-translational modifications of hsp70 promoter in cultured cortical neurons: implications in the heat-shock response. PLoS One. 2015; 10:e0129329. PMID: 26053851.
Article14. Verma P, Pfister JA, Mallick S, D'Mello SR. HSF1 protects neurons through a novel trimerization- and HSP-independent mechanism. J Neurosci. 2014; 34:1599–1612. PMID: 24478344.
Article15. Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci U S A. 2011; 108:18378–18383. PMID: 22042860.
Article16. Kang MJ, Yun HH, Lee JH. KRIBB11 accelerates Mcl-1 degradation through an HSF1-independent, Mule-dependent pathway in A549 non-small cell lung cancer cells. Biochem Biophys Res Commun. 2017; 492:304–309. PMID: 28859986.
Article17. Chen K, Qian W, Li J, Jiang Z, Cheng L, Yan B, et al. Loss of AMPK activation promotes the invasion and metastasis of pancreatic cancer through an HSF1-dependent pathway. Mol Oncol. 2017; 11:1475–1492. PMID: 28783244.
Article18. Wan T, Shao J, Hu B, Liu G, Luo P, Zhou Y. Prognostic role of HSF1 overexpression in solid tumors: a pooled analysis of 3,159 patients. Onco Targets Ther. 2018; 11:383–393. PMID: 29398920.
Article19. La SH, Kim SJ, Kang HG, Lee HW, Chun KH. Ablation of human telomerase reverse transcriptase (hTERT) induces cellular senescence in gastric cancer through a galectin-3 dependent mechanism. Oncotarget. 2016; 7:57117–57130. PMID: 27494887.
Article20. Kim SJ, Wang YG, Lee HW, Kang HG, La SH, Choi IJ, et al. Up-regulation of neogenin-1 increases cell proliferation and motility in gastric cancer. Oncotarget. 2014; 5:3386–3398. PMID: 24930499.
Article21. Szász AM, Lánczky A, Nagy Á, Förster S, Hark K, Green JE, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016; 7:49322–49333. PMID: 27384994.
Article22. Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998; 94:471–480. PMID: 9727490.
Article23. Dai C. The heat-shock, or HSF1-mediated proteotoxic stress, response in cancer: from proteomic stability to oncogenesis. Philos Trans R Soc Lond B Biol Sci. 2018; 373:20160525. PMID: 29203710.
Article24. Wang RE. Targeting heat shock proteins 70/90 and proteasome for cancer therapy. Curr Med Chem. 2011; 18:4250–4264. PMID: 21838681.25. Jego G, Hazoumé A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Lett. 2013; 332:275–285. PMID: 21078542.
Article26. Li Q, Feldman RA, Radhakrishnan VM, Carey S, Martinez JD. Hsf1 is required for the nuclear translocation of p53 tumor suppressor. Neoplasia. 2008; 10:1138–1145. PMID: 18813348.
Article27. Sharma A, Meena AS, Bhat MK. Hyperthermia-associated carboplatin resistance: differential role of p53, HSF1 and Hsp70 in hepatoma cells. Cancer Sci. 2010; 101:1186–1193. PMID: 20180806.
Article28. Cigliano A, Wang C, Pilo MG, Szydlowska M, Brozzetti S, Latte G, et al. Inhibition of HSF1 suppresses the growth of hepatocarcinoma cell lines in vitro and AKT-driven hepatocarcinogenesis in mice. Oncotarget. 2017; 8:54149–54159. PMID: 28903330.
Article29. Gökmen-Polar Y, Badve S. Upregulation of HSF1 in estrogen receptor positive breast cancer. Oncotarget. 2016; 7:84239–84245. PMID: 27713164.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathological Significance of p53 and HSP27 in Gastric-cancer Patients
- Prognostic Significance of Heat Shock Protein 70 Expression in Early Gastric Carcinoma
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- The Role of Heat Shock Protein in Perinatal Fields