Yonsei Med J.
2017 Jul;58(4):807-815. 10.3349/ymj.2017.58.4.807.
Cortical Thickness and White Matter Integrity are Associated with CTG Expansion Size in Myotonic Dystrophy Type I
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation and Hallym Institute of Translational Genomics and Bioinformatics, Hallym Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 2Department of Rehabilitation Medicine and Rehabilitation Institute of Neuromuscular Disease, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. drtlc@yuhs.ac
- 3Department of Neurology and Rehabilitation Institute of Neuromuscular Disease, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2419088
- DOI: http://doi.org/10.3349/ymj.2017.58.4.807
Abstract
- PURPOSE
Myotonic dystrophy type 1 (DM1) is characterized by progressive muscular weakness with symptoms caused by involvement of the brain. The aim of this study was to delineate global changes in cortical thickness and white matter integrity in patients with DM1, compared to age-matched healthy controls, and in brain areas highly correlated with CTG repeat size.
MATERIALS AND METHODS
Cortical thickness and white matter integrity were compared in nine adult DM1 patients and age matched healthy controls using T1-weighted and diffusion tensor imaging. The patients' intelligence quotient (IQ) and CTG repeat size were measured in each individual.
RESULTS
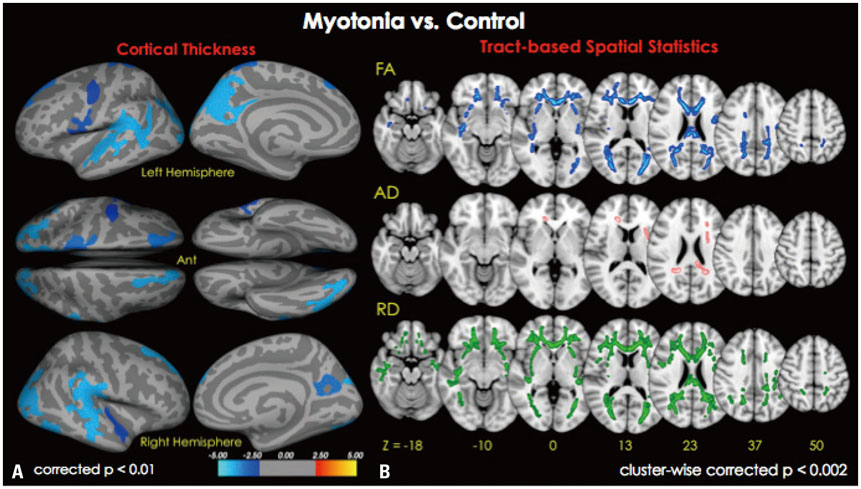
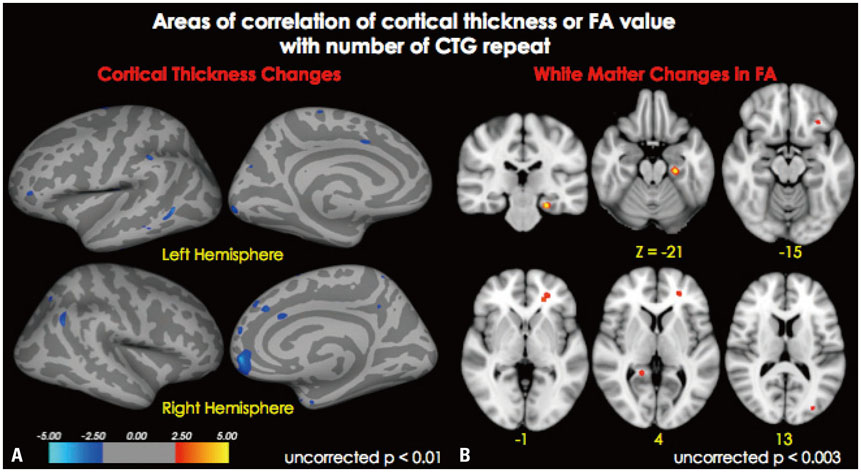
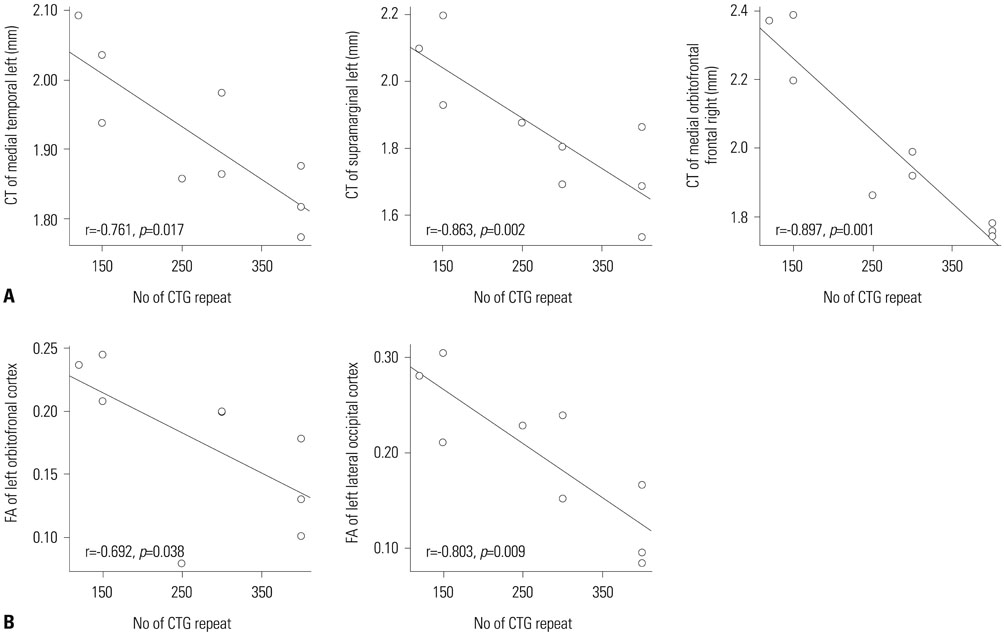
Cortical thickness was significantly reduced in the frontal, temporal, and occipital cortices, while tract-based spatial statistics showed decreased diffusion metrics in widespread areas, including the bilateral orbitofrontal, anterior frontal, insular, external capsule, and occipital cortices in DM1 patients, compared to controls. Additionally, thickness was negatively correlated with the number of CTG repeats in those areas. White matter integrity was negatively correlated with CTG repeats in the left entorhinal, anterior corona radiata, orbitofrontal, and lateral occipital areas. No statistically significant correlation was found between IQ scores and the size of CTG repeats.
CONCLUSION
Our results suggest that DM1 is associated with wide distributions of network changes in both gray and white matter. Some of areas related to cognition showed significant correlations with CTG repeats.
Keyword
MeSH Terms
Figure
Reference
-
1. Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992; 69:385.
Article2. Huber SJ, Kissel JT, Shuttleworth EC, Chakeres DW, Clapp LE, Brogan MA. Magnetic resonance imaging and clinical correlates of intellectual impairment in myotonic dystrophy. Arch Neurol. 1989; 46:536–540.
Article3. Meola G, Sansone V, Perani D, Colleluori A, Cappa S, Cotelli M, et al. Reduced cerebral blood flow and impaired visual-spatial function in proximal myotonic myopathy. Neurology. 1999; 53:1042–1050.
Article4. Modoni A, Silvestri G, Pomponi MG, Mangiola F, Tonali PA, Marra C. Characterization of the pattern of cognitive impairment in myotonic dystrophy type 1. Arch Neurol. 2004; 61:1943–1947.
Article5. Meola G, Sansone V, Perani D, Scarone S, Cappa S, Dragoni C, et al. Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM-1) and in proximal myotonic myopathy (PROMM/DM-2). Neuromuscul Disord. 2003; 13:813–821.
Article6. Meola G, Sansone V. Cerebral involvement in myotonic dystrophies. Muscle Nerve. 2007; 36:294–306.
Article7. Chang L, Anderson T, Migneco OA, Boone K, Mehringer CM, Villanueva-Meyer J, et al. Cerebral abnormalities in myotonic dystrophy. Cerebral blood flow, magnetic resonance imaging, and neuropsychological tests. Arch Neurol. 1993; 50:917–923.8. Antonini G, Mainero C, Romano A, Giubilei F, Ceschin V, Gragnani F, et al. Cerebral atrophy in myotonic dystrophy: a voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2004; 75:1611–1613.
Article9. Ota M, Sato N, Ohya Y, Aoki Y, Mizukami K, Mori T, et al. Relationship between diffusion tensor imaging and brain morphology in patients with myotonic dystrophy. Neurosci Lett. 2006; 407:234–239.
Article10. Kroksmark AK, Ekström AB, Björck E, Tulinius M. Myotonic dystrophy: muscle involvement in relation to disease type and size of expanded CTG-repeat sequence. Dev Med Child Neurol. 2005; 47:478–485.
Article11. Arsenault ME, Prévost C, Lescault A, Laberge C, Puymirat J, Mathieu J. Clinical characteristics of myotonic dystrophy type 1 patients with small CTG expansions. Neurology. 2006; 66:1248–1250.
Article12. Winblad S, Lindberg C, Hansen S. Cognitive deficits and CTG repeat expansion size in classical myotonic dystrophy type 1 (DM1). Behav Brain Funct. 2006; 2:16.13. Angeard N, Gargiulo M, Jacquette A, Radvanyi H, Eymard B, Héron D. Cognitive profile in childhood myotonic dystrophy type 1: is there a global impairment? Neuromuscul Disord. 2007; 17:451–458.
Article14. Kuo HC, Hsieh YC, Wang HM, Chuang WL, Huang CC. Correlation among subcortical white matter lesions, intelligence and CTG repeat expansion in classic myotonic dystrophy type 1. Acta Neurol Scand. 2008; 117:101–107.
Article15. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000; 97:11050–11055.
Article16. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006; 31:1487–1505.
Article17. Wozniak JR, Mueller BA, Bell CJ, Muetzel RL, Lim KO, Day JW. Diffusion tensor imaging reveals widespread white matter abnormalities in children and adolescents with myotonic dystrophy type 1. J Neurol. 2013; 260:1122–1131.
Article18. Endo A, Motonaga K, Arahata K, Harada K, Yamada T, Takashima S. Developmental expression of myotonic dystrophy protein kinase in brain and its relevance to clinical phenotype. Acta Neuropathol. 2000; 100:513–520.
Article19. Di Costanzo A, Di Salle F, Santoro L, Tessitore A, Bonavita V, Tedeschi G. Pattern and significance of white matter abnormalities in myotonic dystrophy type 1: an MRI study. J Neurol. 2002; 249:1175–1182.
Article20. Tanabe Y, Iai M, Tamai K, Fujimoto N, Sugita K. Neuroradiological findings in children with congenital myotonic dystrophy. Acta Paediatr. 1992; 81:613–617.
Article21. Minnerop M, Weber B, Schoene-Bake JC, Roeske S, Mirbach S, Anspach C, et al. The brain in myotonic dystrophy 1 and 2: evidence for a predominant white matter disease. Brain. 2011; 134(Pt 12):3530–3546.
Article22. Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008; 105:18035–18040.
Article23. Ogata A, Terae S, Fujita M, Tashiro K. Anterior temporal white matter lesions in myotonic dystrophy with intellectual impairment: an MRI and neuropathological study. Neuroradiology. 1998; 40:411–415.
Article24. Giorgio A, Dotti MT, Battaglini M, Marino S, Mortilla M, Stromillo ML, et al. Cortical damage in brains of patients with adult-form of myotonic dystrophy type 1 and no or minimal MRI abnormalities. J Neurol. 2006; 253:1471–1477.
Article25. Bungener C, Jouvent R, Delaporte C. Psychopathological and emotional deficits in myotonic dystrophy. J Neurol Neurosurg Psychiatry. 1998; 65:353–356.
Article26. Delaporte C. Personality patterns in patients with myotonic dystrophy. Arch Neurol. 1998; 55:635–640.
Article27. Goossens E, Steyaert J, De Die-Smulders C, Willekens D, Fryns JP. Emotional and behavioral profile and child psychiatric diagnosis in the childhood type of myotonic dystrophy. Genet Couns. 2000; 11:317–327.28. Perini GI, Menegazzo E, Ermani M, Zara M, Gemma A, Ferruzza E, et al. Cognitive impairment and (CTG)n expansion in myotonic dystrophy patients. Biol Psychiatry. 1999; 46:425–431.
Article29. Censori B, Provinciali L, Danni M, Chiaramoni L, Maricotti M, Foschi N, et al. Brain involvement in myotonic dystrophy: MRI features and their relationship to clinical and cognitive conditions. Acta Neurol Scand. 1994; 90:211–217.
Article30. Winblad S, Lindberg C, Hansen S. Temperament and character in patients with classical myotonic dystrophy type 1 (DM-1). Neuromuscul Disord. 2005; 15:287–292.
Article31. Storsve AB, Fjell AM, Yendiki A, Walhovd KB. Longitudinal changes in white matter tract integrity across the adult lifespan and its relation to cortical thinning. PLoS One. 2016; 11:e0156770.
Article32. Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, Jansen G, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992; 255:1253–1255.
Article33. Hunter A, Tsilfidis C, Mettler G, Jacob P, Mahadevan M, Surh L, et al. The correlation of age of onset with CTG trinucleotide repeat amplification in myotonic dystrophy. J Med Genet. 1992; 29:774–779.
Article34. Harley HG, Rundle SA, Reardon W, Myring J, Crow S, Brook JD, et al. Unstable DNA sequence in myotonic dystrophy. Lancet. 1992; 339:1125–1128.
Article35. Redman JB, Fenwick RG Jr, Fu YH, Pizzuti A, Caskey CT. Relationship between parental trinucleotide GCT repeat length and severity of myotonic dystrophy in offspring. JAMA. 1993; 269:1960–1965.
Article36. Weber YG, Roebling R, Kassubek J, Hoffmann S, Rosenbohm A, Wolf M, et al. Comparative analysis of brain structure, metabolism, and cognition in myotonic dystrophy 1 and 2. Neurology. 2010; 74:1108–1117.
Article37. Huguet A, Medja F, Nicole A, Vignaud A, Guiraud-Dogan C, Ferry A, et al. Molecular, physiological, and motor performance defects in DMSXL mice carrying >1,000 CTG repeats from the human DM1 locus. PLoS Genet. 2012; 8:e1003043.
Article38. Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996; 16:5233–5255.
Article39. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001; 98:676–682.
Article40. Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000; 123(Pt 11):2189–2202.
Article41. Simpson JR Jr, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci U S A. 2001; 98:683–687.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The CTG Repeat Polymorphisms of Myotonic Dystrophy (DM) Gene in Korean Population
- Clinical Significance of CTG Repeat Expansion in Korean Myotonic Dystrophy Patients
- Bilateral Adduction Palsy in a Patient with Myotonic Dystrophy Type 1
- Patterns of Brain Lesions in Adult-Onset Myotonic Dystrophy Type 1: A Quantitative MRI Study
- A Case of Congenital Myotonic Dystrophy Diagnosed by Molecular Genetics