Yonsei Med J.
2017 Nov;58(6):1160-1169. 10.3349/ymj.2017.58.6.1160.
Identification and Functional Characterization of ST3GAL5 and ST8SIA1 Variants in Patients with Thyroid-Associated Ophthalmopathy
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Ewha Womans University, Seoul, Korea. jihachoi@ewha.ac.kr
- 2Tissue Injury Defense Research Center, College of Medicine, Ewha Womans University, Seoul, Korea.
- 3Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Physiology, College of Medicine, Ewha Womans University, Seoul, Korea.
- 5Department of Ophthalmology, Ajou University School of Medicine, Suwon, Korea. drkook@ajou.ac.kr
- KMID: 2418898
- DOI: http://doi.org/10.3349/ymj.2017.58.6.1160
Abstract
- PURPOSE
This study was conducted to identify and to functionally characterize genetic variants in ST3GAL5 and ST8SIA1 in Korean patients with thyroid-associated ophthalmopathy (TAO).
MATERIALS AND METHODS
Genetic analyses were conducted using DNA samples from TAO patients (n=50) and healthy subjects (n=48) to identify TAO-specific genetic variants of ST3GAL5 or ST8SIA1. The effect of each genetic variant on the transcription or expression of these genes was examined. Additionally, correlations between functional haplotypes of ST3GAL5 or ST8SIA1 and clinical characteristics of the patients were investigated.
RESULTS
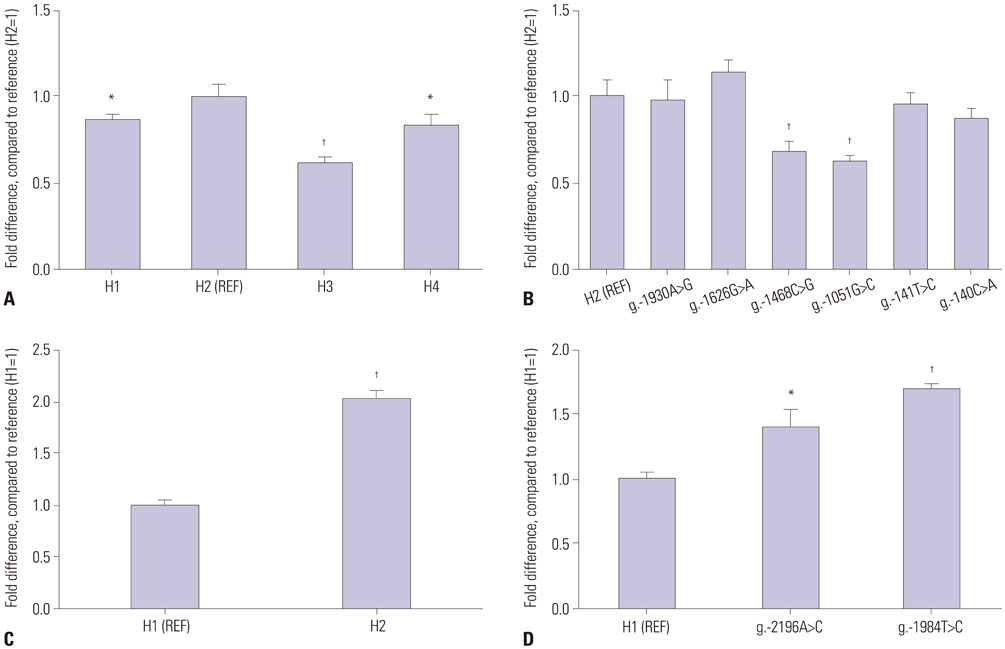
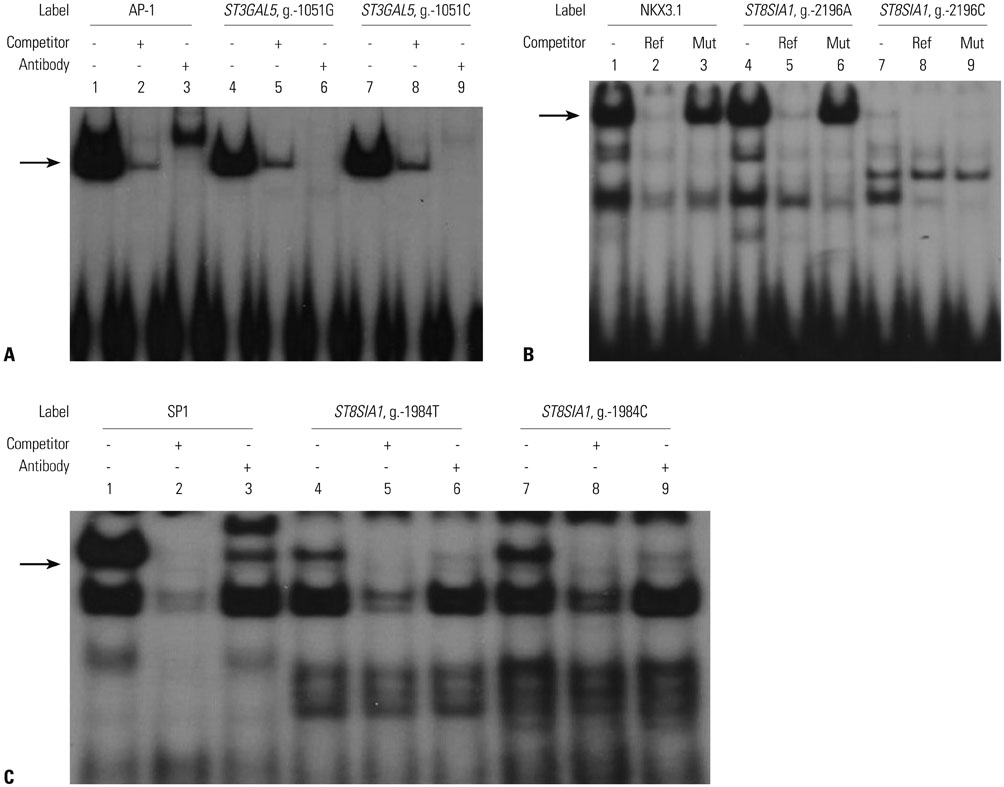
Six promoter variants and one nonsynonymous variant of ST3GAL5 were identified, and four major promoter haplotypes were assembled. Additionally, three promoter variants and two major haplotypes of ST8SIA1 were identified. All ST3GAL5 and ST8SIA1 variants identified in TAO patients were also found in healthy controls. Promoter activity was significantly decreased in three promoter haplotypes of ST3GAL5 and increased in one promoter haplotype of ST8SIA1. Transcription factors activating protein-1, NKX3.1, and specificity protein 1 were revealed as having roles in transcriptional regulation of these haplotypes. The nonsynonymous variant of ST3GAL5, H104R, did not alter the expression of ST3GAL5. While no differences in clinical characteristics were detected in patients possessing the functional promoter haplotypes of ST3GAL5, exophthalmic values were significantly lower in patients with the ST8SIA1 haplotype, which showed a significant increase in promoter activity.
CONCLUSION
These results from genotype-phenotype analysis might suggest a possible link between the ST8SIA1 functional promoter haplotype and the clinical severity of TAO. However, further studies with larger sample sizes are warranted.
Keyword
MeSH Terms
-
Adult
Case-Control Studies
Female
Gene Expression Regulation
Genetic Variation
Graves Ophthalmopathy/ethnology/*genetics
Haplotypes
Humans
Male
Middle Aged
Phenotype
Polymorphism, Single Nucleotide
Promoter Regions, Genetic
Sialyltransferases/*genetics/metabolism
Transcription Factors
Transcription Factors
Sialyltransferases
Figure
Reference
-
1. Kuriyan AE, Phipps RP, Feldon SE. The eye and thyroid disease. Curr Opin Ophthalmol. 2008; 19:499–506.
Article2. RUNDLE FF. Management of exophthalmos and related ocular changes in Graves' disease. Metabolism. 1957; 6:36–48.3. Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid. 2002; 12:855–860.
Article4. Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010; 362:726–738.
Article5. Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev. 2000; 21:168–199.
Article6. Liu YH, Chen CC, Liao LL, Wan L, Tsai CH, Tsai FJ. Association of IL12B polymorphisms with susceptibility to Graves ophthalmopathy in a Taiwan Chinese population. J Biomed Sci. 2012; 19:97.
Article7. Liu YH, Chen RH, Wu HH, Liao WL, Chen WC, Tsai Y, et al. Association of interleukin-1β (IL1B) polymorphisms with Graves' ophthalmopathy in Taiwan Chinese patients. Invest Ophthalmol Vis Sci. 2010; 51:6238–6246.
Article8. Niyazoglu M, Baykara O, Koc A, Aydogğdu P, Onaran I, Dellal FD, et al. Association of PARP-1, NF-κB, NF-κBIA and IL-6, IL-1β and TNF-α with Graves disease and Graves ophthalmopathy. Gene. 2014; 547:226–232.
Article9. Liao WL, Chen RH, Lin HJ, Liu YH, Chen WC, Tsai Y, et al. Toll-like receptor gene polymorphisms are associated with susceptibility to Graves' ophthalmopathy in Taiwan males. BMC Med Genet. 2010; 11:154.
Article10. van Echten G, Sandhoff K. Ganglioside metabolism. Enzymology, topology, and regulation. J Biol Chem. 1993; 268:5341–5344.
Article11. Kook KH, Choi YH, Kim YR, Park SJ, Jou I, Kim SJ, et al. Altered ganglioside expression modulates the pathogenic mechanism of thyroid-associated ophthalmopathy by increase in hyaluronic acid. Invest Ophthalmol Vis Sci. 2011; 52:264–273.
Article12. Kaback LA, Smith TJ. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1β in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1999; 84:4079–4084.
Article13. Werner SC. Classification of the eye changes of Grave's disease. J Clin Endocrinol Metab. 1969; 29:982–984.
Article14. Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf). 1997; 47:9–14.
Article15. Lam AK, Lam CF, Leung WK, Hung PK. Intra-observer and inter-observer variation of Hertel exophthalmometry. Ophthalmic Physiol Opt. 2009; 29:472–476.
Article16. Choi JH, Yee SW, Kim MJ, Nguyen L, Lee JH, Kang JO, et al. Identification and characterization of novel polymorphisms in the basal promoter of the human transporter, MATE1. Pharmacogenet Genomics. 2009; 19:770–780.
Article17. Choi JH, Yee SW, Ramirez AH, Morrissey KM, Jang GH, Joski PJ, et al. A common 5'-UTR variant in MATE2-K is associated with poor response to metformin. Clin Pharmacol Ther. 2011; 90:674–684.
Article18. Jang GH, Kim TH, Choe Y, Ham A, Choi JH. Functional characterization of genetic variations in the MDR3 promoter. Biochem Biophys Res Commun. 2013; 430:1312–1318.
Article19. Tsuji S. Molecular cloning and functional analysis of sialyltransferases. J Biochem. 1996; 120:1–13.
Article20. Proia RL. Gangliosides help stabilize the brain. Nat Genet. 2004; 36:1147–1148.
Article21. Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A. 2003; 100:3445–3449.
Article22. Yoshikawa M, Go S, Takasaki K, Kakazu Y, Ohashi M, Nagafuku M, et al. Mice lacking ganglioside GM3 synthase exhibit complete hearing loss due to selective degeneration of the organ of Corti. Proc Natl Acad Sci U S A. 2009; 106:9483–9488.
Article23. Simpson MA, Cross H, Proukakis C, Priestman DA, Neville DC, Reinkensmeier G, et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet. 2004; 36:1225–1229.
Article24. Fragaki K, Ait-El-Mkadem S, Chaussenot A, Gire C, Mengual R, Bonesso L, et al. Refractory epilepsy and mitochondrial dysfunction due to GM3 synthase deficiency. Eur J Hum Genet. 2013; 21:528–534.
Article25. Husain S, Yildirim-Toruner C, Rubio JP, Field J, Schwalb M, et al. Southern MS Genetics Consortium. Variants of ST8SIA1 are associated with risk of developing multiple sclerosis. PLoS One. 2008; 3:e2653.
Article26. Ramagopalan SV, Morrison KM, Para A, Handel A, Disanto G, Handunnetthi L, et al. Variants in ST8SIA1 do not play a major role in susceptibility to multiple sclerosis in Canadian families. J Neuroimmunol. 2009; 212:142–144.
Article27. Kim SW, Lee SH, Kim KS, Kim CH, Choo YK, Lee YC. Isolation and characterization of the promoter region of the human GM3 synthase gene. Biochim Biophys Acta. 2002; 1578:84–89.
Article28. Zeng G, Gao L, Xia T, Tencomnao T, Yu RK. Characterization of the 5'-flanking fragment of the human GM3-synthase gene. Biochim Biophys Acta. 2003; 1625:30–35.
Article29. Chung TW, Choi HJ, Lee YC, Kim CH. Molecular mechanism for transcriptional activation of ganglioside GM3 synthase and its function in differentiation of HL-60 cells. Glycobiology. 2005; 15:233–244.
Article30. Ozanne BW, Spence HJ, McGarry LC, Hennigan RF. Transcription factors control invasion: AP-1 the first among equals. Oncogene. 2007; 26:1–10.
Article31. Pfäfflin A, Brodbeck K, Heilig CW, Häring HU, Schleicher ED, Weigert C. Increased glucose uptake and metabolism in mesangial cells overexpressing glucose transporter 1 increases interleukin-6 and vascular endothelial growth factor production: role of AP-1 and HIF-1α. Cell Physiol Biochem. 2006; 18:199–210.
Article32. Duane WC, Xiong W, Lofgren J. Transactivation of the human apical sodium-dependent bile acid transporter gene by human serum. J Steroid Biochem Mol Biol. 2008; 108:137–148.
Article33. Ramírez-Sotelo G, López-Bayghen E, Hernández-Kelly LC, Arias-Montaño JA, Bernabé A, Ortega A. Regulation of the mouse Na+-dependent glutamate/aspartate transporter GLAST: putative role of an AP-1 DNA binding site. Neurochem Res. 2007; 32:73–80.
Article34. Mittelstadt ML, Patel RC. AP-1 mediated transcriptional repression of matrix metalloproteinase-9 by recruitment of histone deacetylase 1 in response to interferon β. PLoS One. 2012; 7:e42152.
Article35. Kang NY, Kang SK, Lee YC, Choi HJ, Lee YS, Cho SY, et al. Transcriptional regulation of the human GD3 synthase gene expression in Fas-induced Jurkat T cells: a critical role of transcription factor NF-κB in regulated expression. Glycobiology. 2006; 16:375–389.
Article36. Martinez EE, Darke AK, Tangen CM, Goodman PJ, Fowke JH, Klein EA, et al. A functional variant in NKX31 associated with prostate cancer risk in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Cancer Prev Res (Phila). 2014; 7:950–957.
Article37. Song LN, Silva J, Koller A, Rosenthal A, Chen EI, Gelmann EP. The tumor suppressor NKX3.1 is targeted for degradation by DYRK1B kinase. Mol Cancer Res. 2015; 13:913–922.
Article38. Solomon SS, Majumdar G, Martinez-Hernandez A, Raghow R. A critical role of Sp1 transcription factor in regulating gene expression in response to insulin and other hormones. Life Sci. 2008; 83:305–312.
Article39. Samson SL, Wong NC. Role of Sp1 in insulin regulation of gene expression. J Mol Endocrinol. 2002; 29:265–279.
Article40. Champigny MJ, Johnson M, Igdoura SA. Characterization of the mouse lysosomal sialidase promoter. Gene. 2003; 319:177–187.
Article41. Lim HS, Back KO, Kim HJ, Choi YH, Park YM, Kook KH. Hyaluronic acid induces COX-2 expression via CD44 in orbital fibroblasts from patients with thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014; 55:7441–7450.
Article42. Han R, Smith TJ. T helper type 1 and type 2 cytokines exert divergent influence on the induction of prostaglandin E2 and hyaluronan synthesis by interleukin-1β in orbital fibroblasts: implications for the pathogenesis of thyroid-associated ophthalmopathy. Endocrinology. 2006; 147:13–19.
Article43. Bobowski M, Vincent A, Steenackers A, Colomb F, Van Seuningen I, Julien S, et al. Estradiol represses the GD3 synthase gene ST8SIA1 expression in human breast cancer cells by preventing NFκB binding to ST8SIA1 promoter. PLoS One. 2013; 8:e62559.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Manifestations of Thyroid-associated Ophthalmopathy Accompanied by Thyroid Cancer
- The Effect of Previous Orbital Decompression on Outcome of Strabismus Surgery in Patients with Thyroid Ophthalmopathy
- Strabismus Surgery for Thyroid Ophthalmopathy
- Current Therapy of Thyroid-associated Ophthalmopathy
- Central Retinal Artery Occlusion in Association with Thyroid Ophthalmopathy