J Vet Sci.
2017 Mar;18(1):67-71. 10.4142/jvs.2017.18.1.67.
Surgical access via right thoracotomy facilitates tricuspid valve surgery in sheep
- Affiliations
-
- 1Department of Cardiothoracic Surgery, Jena University Hospital, Friedrich Schiller University, Jena 07747, Germany. doenst@med.uni-jena.de
- 2Department of Cardiovascular Surgery, Heart Center, University of Freiburg, Freiburg 79110, Germany.
- 3Institute of Laboratory Animals Science and Welfare, Jena University Hospital, Friedrich Schiller University, Jena 07747, Germany.
- KMID: 2412589
- DOI: http://doi.org/10.4142/jvs.2017.18.1.67
Abstract
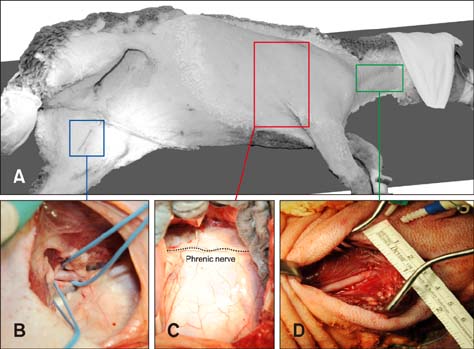
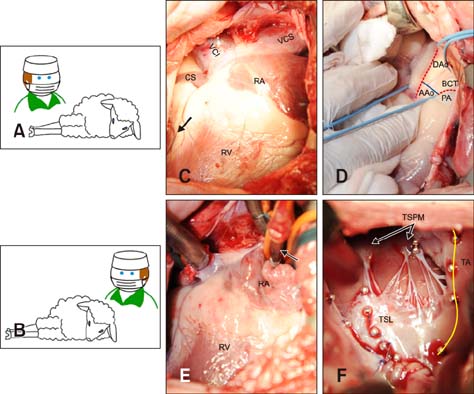
- In quadrupeds, the three-dimensional orientation of the heart with respect to the thorax is fundamentally different from that in humans. In this study, we assessed the best surgical approach to the tricuspid valve in sheep. Firstly, different surgical access sites to the tricuspid valve were tested in sheep cadavers, the anatomy was analyzed, and the optimal surgical approach to the tricuspid valve was determined. Secondly - along with cardiopulmonary bypass and cardioplegic arrest -the chosen approach was tested in six adult sheep in vivo. Anatomical analyses revealed that a left thoracotomy provided optimal access to the aorta and left heart. However, visualization of the right heart was significantly impaired. In contrast, a right thoracotomy provided good access to the right heart, but the ascending aorta was difficult to approach. Therefore, in the in vivo studies, arterial cannulation was performed through a carotid (n = 4) or femoral (n = 2) artery. In conclusion, a right-sided thoracotomy allows good visualization of all components of the tricuspid valve complex in sheep, but not of the ascending aorta. Consequently, peripheral vessels are preferred for arterial cannulation. This work may stimulate the investigation of pathomechanisms and/or novel treatment options for tricuspid valve pathologies.
Keyword
Figure
Reference
-
1. Bothe W, Chang PA, Swanson JC, Itoh A, Arata K, Ingels NB, Miller DC. Releasable annuloplasty ring insertion—a novel experimental implantation model. Eur J Cardiothorac Surg. 2009; 36:830–832.
Article2. Bothe W, Kuhl E, Kvitting JPE, Rausch MK, Göktepe S, Swanson JC, Farahmandnia S, Ingels NB Jr, Miller DC. Rigid, complete annuloplasty rings increase anterior mitral leaflet strains in the normal beating ovine heart. Circulation. 2011; 124:11 Suppl. S81–S96.
Article3. Gilbert JW Jr, Mansour K, Sanders S, Gravanis MB. Experimental reconstruction of the tricuspid valve with autologous fascia lata. Arch Surg. 1968; 97:149–153.
Article4. Glasson JR, Komeda M, Daughters GT, Bolger AF, Karlsson MO, Foppiano LE, Hayase M, Oesterle SN, Ingels NB Jr, Miller DC. Early systolic mitral leaflet “loitering” during acute ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 1998; 116:193–205.
Article5. Hendry PJ, Ascah KJ, Rajagopalan K, Calvin JE. Does septal position affect right ventricular function during left ventricular assist in an experimental porcine model? Circulation. 1994; 90(5 Pt 2):II353–II358.6. Kinney TE, Olinger GN, Sagar KB, Boerboom LE. Acute, reversible tricuspid insufficiency: creation in canine model. Am J Physiol. 1991; 260:H638–H641.
Article7. Lai DT, Tibayan FA, Myrmel T, Timek TA, Dagum P, Daughters GT, Liang D, Ingels NB Jr, Miller DC. Mechanistic insights into posterior mitral leaflet inter-scallop malcoaptation during acute ischemic mitral regurgitation. Circulation. 2002; 106:12 Suppl 1. I40–I45.
Article8. Timek TA, Dagum P, Lai DT, Liang D, Daughters GT, Tibayan F, Ingels NB Jr, Miller DC. Tachycardia-induced cardiomyopathy in the ovine heart: mitral annular dynamic three-dimensional geometry. J Thorac Cardiovasc Surg. 2003; 125:315–324.
Article9. Walter EMD, Vasilyev NV, Sill B, Padala M, Jimenez J, Yoganathan AP, Hetzer R, del Nido PJ. Creation of a tricuspid valve regurgitation model from tricuspid annular dilatation using the cardioport video-assisted imaging system. J Heart Valve Dis. 2011; 20:184–188.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tricuspid Replacement through Right Thoracotomy in Reoperation: A case report
- Permanent Pacemaker Lead Induced Severe Tricuspid Regurgitation in Patient Undergoing Multiple Valve Surgery
- Tricuspid Valve Re-Repair in Ebstein Anomaly Using the Cone Technique
- A Case Report of Surgical Management of Tricuswpid Valve Endocarditis
- Cone Reconstruction for Tricuspid Valve Repair in a Patient with Ebstein's Anomaly : A case report