J Vet Sci.
2017 Jun;18(2):253-256. 10.4142/jvs.2017.18.2.253.
Duplex nested reverse transcriptase polymerase chain reaction for simultaneous detection of type 2 porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 from tissue samples
- Affiliations
-
- 1Infectious Disease Research Center, Korea Research Institute of Bioscience & Biotechnology, Daejeon 34141, Korea.
- 2Korea Zoonosis Research Institute, Chonbuk National University, Jeonju 54596, Korea.
- 3Department of Veterinary Microbiology-Infectious Diseases, Faculty of Veterinary Medicine, Vietnam National University of Agriculture, Trau Quy, Gia Lam, Hanoi, Vietnam.
- 4Research Unit, Green Cross Veterinary Products, Yongin 17066, Korea.
- 5Department of Veterinary Medicine Virology Lab, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. parkx026@snu.ac.kr
- KMID: 2412579
- DOI: http://doi.org/10.4142/jvs.2017.18.2.253
Abstract
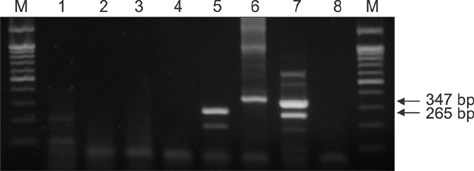
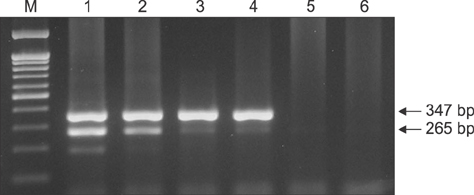
- There are high levels of co-incidence of porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2) in porcine tissue. This study established a duplex nested reverse transcriptase polymerase chain reaction (RT-PCR) method that targets the genomic RNA of type 2 PRRSV and the mRNA of PCV2 in infected tissues. The method amplified discriminative bands of 347 bp and 265 bp specific for type 2 PRRSV and PCV2, respectively. The limits of detection of the duplex nested RT-PCR were 10(1.5) TCIDâ‚…â‚€/mL for type 2 PRRSV and 10² infected cells/mL for PCV2. The kappa statistic, which measures agreement between methods, was 0.867, indicating a good level of agreement. This RNA-based duplex RT-PCR approach can be another way to detect type 2 PRRSV and PCV2 simultaneously and with improved convenience.
Keyword
MeSH Terms
-
Animals
Circoviridae Infections/*diagnosis/virology
Circovirus/*genetics
Coinfection/diagnosis/*veterinary/virology
Limit of Detection
Polymerase Chain Reaction/methods/*veterinary
Porcine Reproductive and Respiratory Syndrome/*diagnosis/virology
Porcine respiratory and reproductive syndrome virus/*genetics
Swine
Swine Diseases/*diagnosis/virology
Figure
Reference
-
1. Allan GM, McNeilly F, Ellis J, Krakowka S, Meehan B, McNair I, Walker I, Kennedy S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch Virol. 2000; 145:2421–2429.
Article2. Ellis J, Hassard L, Clark E, Harding J, Allan G, Willson P, Strokappe J, Martin K, McNeilly F, Meehan B, Todd D, Haines D. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can Vet J. 1998; 39:44–51.3. Ellis J, Krakowka S, Lairmore M, Haines D, Bratanich A, Clark E, Allan G, Konoby C, Hassard L, Meehan B, Martin K, Harding J, Kennedy S, McNeilly F. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. J Vet Diagn Invest. 1999; 11:3–14.
Article4. Giammarioli M, Pellegrini C, Casciari C, De Mia GM. Development of a novel hot-start multiplex PCR for simultaneous detection of classical swine fever virus, African swine fever virus, porcine circovirus type 2, porcine reproductive and respiratory syndrome virus and porcine parvovirus. Vet Res Commun. 2008; 32:255–262.
Article5. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of 2 US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995; 32:648–660.
Article6. Harms PA, Sorden SD, Halbur PG, Bolin SR, Lager KM, Morozov I, Paul PS. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol. 2001; 38:528–539.
Article7. Kennedy S, Moffett D, McNeilly F, Meehan B, Ellis J, Krakowka S, Allan GM. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J Comp Pathol. 2000; 122:9–24.
Article8. Kim J, Chung HK, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. 2003; 166:251–256.
Article9. Kono Y, Kanno T, Shimizu M, Yamada S, Ohashi S, Nakamine M, Shirai J. Nested PCR for detection and typing of porcine reproductive and respiratory syndrome (PRRS) virus in pigs. J Vet Med Sci. 1996; 58:941–946.
Article10. Murphy FA, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis A, Martelli GP, Mayo MA, Summers MD. Virus Taxonomy. New York: Springer;1995.11. Pogranichniy RM, Yoon KJ, Harms PA, Sorden SD, Daniels M. Case-control study on the association of porcine circovirus type 2 and other swine viral pathogens with postweaning multisystemic wasting syndrome. J Vet Diagn Invest. 2002; 14:449–456.
Article12. Rossow KD, Benfield DA, Goyal SM, Nelson EA, Christopher-Hennings J, Collins JE. Chronological immunohistochemical detection and localization of porcine reproductive and respiratory syndrome virus in gnotobiotic pigs. Vet Pathol. 1996; 33:551–556.
Article13. Yang JS, Song DS, Kim SY, Lyoo KS, Park BK. Detection of porcine circovirus type 2 in feces of pigs with or without enteric disease by polymerase chain reaction. J Vet Diagn Invest. 2003; 15:369–373.
Article14. Yu S, Carpenter S, Opriessnig T, Halbur PG, Thacker E. Development of a reverse transcription-PCR assay to detect porcine circovirus type 2 transcription as a measure of replication. J Virol Methods. 2005; 123:109–112.
Article15. Yu S, Opriessnig T, Kitikoon P, Nilubol D, Halbur PG, Thacker E. Porcine circovirus type 2 (PCV2) distribution and replication in tissues and immune cells in early infected pigs. Vet Immunol Immunopathol. 2007; 115:261–272.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2 and porcine parvovirus from aborted fetuses and pigs with respiratory problems in Korea
- Survey of porcine respiratory disease complex-associated pathogens among commercial pig farms in Korea via oral fluid method
- Age-specific Prevalence of Porcine Reproductive and Respiratory Syndrome Virus, Porcine Circovirus Type 2, and Mycoplasma hyopneumoniae in Korea Pig Farms
- Development of a multiplex qRT-PCR assay for detection of African swine fever virus, classical swine fever virus and porcine reproductive and respiratory syndrome virus
- Pathologic studies in lymph nodes of pigs infected with porcine circovirus type 2, porcine reproductive and respiratory syndrome virus