J Vet Sci.
2017 Sep;18(3):341-348. 10.4142/jvs.2017.18.3.341.
Guanylyl cyclase C and guanylin reduce fat droplet accumulation in cattle mesenteric adipose tissue
- Affiliations
-
- 1Department of Veterinary Anatomy, Faculty of Agriculture, University of Miyazaki, Miyazaki 889-2192, Japan. yasudaja@cc.miyazaki-u.ac.jp
- 2Frontier Science Research Center, University of Miyazaki, Miyazaki 889-1692, Japan.
- KMID: 2412452
- DOI: http://doi.org/10.4142/jvs.2017.18.3.341
Abstract
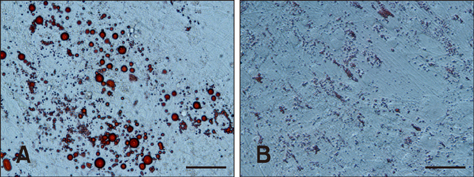
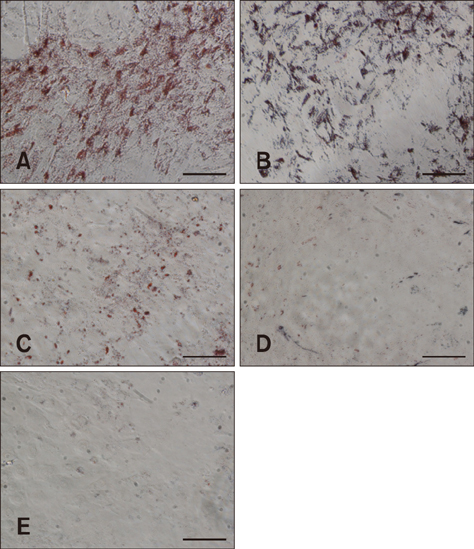
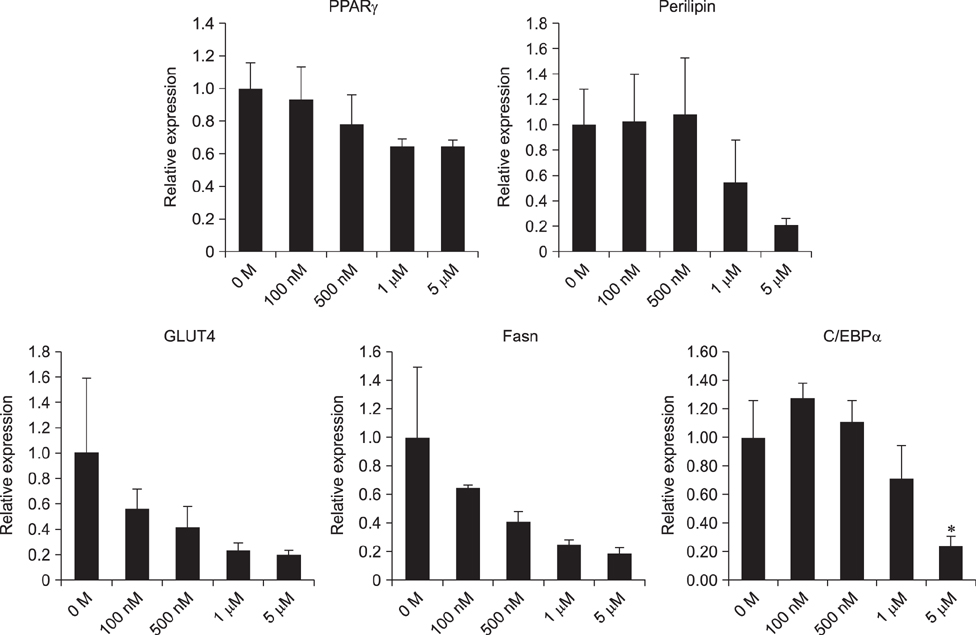
- Guanylyl cyclase C (GC-C) is a member of a family of enzymes that metabolize GTP to cGMP and was first identified as a receptor for heat-stable enterotoxin. Guanylin (GNY) has since been identified as an endogenous ligand for GC-C in the intestine of several mammalian species. The GNY/GC-C system regulates ion transportation and pH in the mucosa. Recently, it was reported that GC-C and GNY are involved in lipid metabolism in rat mesenteric adipose tissue macrophages. To examine the role of GC-C and GNY in lipid metabolism in cattle, we used a bovine mesenteric adipocyte primary culture system and a coculture system for bovine adipocytes and GNY-/GC-C-expressing macrophages. Fat droplets were observed to accumulate in bovine mesenteric adipocytes cultured alone, whereas few fat droplets accumulated in adipocytes indirectly cocultured with macrophages. We also observed that GC-C was present in bovine mesenteric adipose tissue, and that fat droplet accumulation decreased after in vitro GNY administration. Expressions of mRNAs encoding lipogenic factors decreased significantly in adipocytes after either coculture or GNY administration. These results suggest that the GNY/GC-C system is part of the control system for lipid accumulation in bovine mesenteric adipose tissue.
MeSH Terms
-
Adipocytes/drug effects/metabolism
Adipose Tissue/cytology/*drug effects/metabolism
Animals
Cattle
Coculture Techniques/veterinary
Gastrointestinal Hormones/*pharmacology
Guanylate Cyclase
Lipid Metabolism/drug effects/physiology
Macrophages/drug effects/metabolism
Natriuretic Peptides/*pharmacology
Real-Time Polymerase Chain Reaction/veterinary
Receptors, Enterotoxin/*metabolism
Gastrointestinal Hormones
Natriuretic Peptides
Guanylate Cyclase
Receptors, Enterotoxin
Figure
Reference
-
1. Akieda-Asai S, Sugiyama M, Miyazawa T, Koda S, Okano I, Senba K, Poleni PE, Hizukuri Y, Okamoto A, Yamahara K, Mutoh E, Aoyama F, Sawaguchi A, Furuya M, Miyazato M, Kangawa K, Date Y. Involvement of guanylin and GC-C in rat mesenteric macrophages in resistance to a high-fat diet. J Lipid Res. 2013; 54:85–96.
Article2. Akter SH, Häusser S, Germeroth D, von Soosten D, Dänicke S, Südekum KH, Sauerwein H. Immunohistochemical characterization of phagocytic immune cell infiltration into different adipose tissue depots of dairy cows during early lactation. J Dairy Sci. 2012; 95:3032–3044.
Article3. Al-Majali AM, Asem EK, Lamar CH, Robinson JP, Freeman MJ, Saeed AM. Studies on the mechanism of diarrhoea induced by Escherichia coli heat-stable enterotoxin (STa) in newborn calves. Vet Res Commun. 2000; 24:327–338.4. Arshad N, Visweswariah SS. The multiple and enigmatic roles of guanylyl cyclase C in intestinal homeostasis. FEBS Lett. 2012; 586:2835–2840.
Article5. Carrithers SL, Hill MJ, Johnson BR, O'Hara SM, Jackson BA, Ott CE, Lorenz J, Mann EA, Giannella RA, Forte LR, Greenberg RN. Renal effects of uroguanylin and guanylin in vivo. Braz J Med Biol Res. 1999; 32:1337–1344.6. Cheguru P, Chapalamadugu KC, Doumit ME, Murdoch GK, Hill RA. Adipocyte differentiation-specific gene transcriptional response to C18 unsaturated fatty acids plus insulin. Pflugers Arch. 2012; 463:429–447.
Article7. Contreras GA, Sordillo LM. Lipid mobilization and inflammatory responses during the transition period of dairy caws. Comp Immunol Microbiol Infect Dis. 2011; 34:281–289.
Article8. Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, CE , Smith CE. Guanylin: an endogenouse activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992; 89:947–951.9. de Sauvage FJ, Keshav S, Kuang WJ, Gillett N, Henzel W, Goeddel DV. Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci U S A. 1992; 89:9089–9093.
Article10. Frühbeck G. Gastrointestinal hormones: uroguanylin—a new gut-derived weapon against obesity? Nat Rev Endocrinol. 2012; 8:5–6.11. Gong R, Ding C, Hu J, Lu Y, Liu F, Mann E, Xu F, Cohen MB, Luo M. Role for the membrane receptor guanylyl cyclase-C in attention deficiency and hyperactive behavior. Science. 2011; 333:1642–1646.
Article12. Habegger KM, Hoffman NJ, Ridenour CM, Brozinick JT, Elmendorf JS. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology. 2012; 153:2130–2141.
Article13. Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chi DT, Tompkins JA, Fok KF, Smith CE, Duffin KL, Siegel NR, Currie MG. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1993; 90:10464–10468.
Article14. Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One. 2011; 6:e16139.
Article15. Hasegawa K, Akieda-Asai S, Fujii Y, Bae CR, Yasuda M, Date Y. Guanylin–Guanylyl cyclase-C signaling in macrophages regulates mesenteric fat inflammation induced by high-fat diet. Endocr J. 2015; 62:939–947.
Article16. Inoue K, Honda T, Oyama K. Economic losses related to internal diseases in Japanese Black cattle. Anim Sci J. 2016; 87:736–741.
Article17. Inoue K, Honda T, Oyama K. Genetic relationships between internal diseases diagnosed at slaughter and carcass traits in Japanese Black cattle. J Anim Sci. 2015; 93:2714–2721.
Article18. Kabara E, Sordillo LM, Holcombe S, Contreras GA. Adiponectin links adipose tissue function and monocyte inflammatory responses during bovine metabolic stress. Comp Immunol Microbiol Infect Dis. 2014; 37:49–58.
Article19. Kita T, Smith CE, Fok KF, Duffin KL, Moore WM, Karabatsos PJ, Kachur JF, Hamra FK, Pidhorodeckyj NV, Forte LR, Currie MG. Characterization of human uroguanylin: a member of the guanylin peptide family. Am J Physiol. 1994; 266:F342–F348.
Article20. Kroetz SM, Srinivasan J, Yaghoobian J, Sternberg PW, Hong RL. The cGMP signaling pathway affects feeding behavior in the necromenic nematode Pristionchus pacificus. PLoS One. 2012; 7:e34464.21. Menendez JA, Vazquez-Martin A, Ortega FJ, Fernandez-Real JM. Fatty acid synthase: association with insulin resistance, type 2 diabetes, and cancer. Clin Chem. 2009; 55:425–438.
Article22. Mukesh M, Bionaz M, Graugnard DE, Drackley JK, Loor JJ. Adipose tissue depots of Holstein cows are immune responsive: inflammatory gene expression in vitro. Domest Anim Endocrinol. 2010; 38:168–178.
Article23. Potter LR. Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases. Pharmacol Ther. 2011; 130:71–82.
Article24. Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 2002; 16:22–26.
Article25. Schulz S, Chrisman TD, Garbers DL. Cloning and expression of guanylin. Its existence in various mammalian tissues. J Biol Chem. 1992; 267:16019–16021.
Article26. Shimizu K, Sakai M, Ando M, Chiji H, Kawada T, Mineo H, Taira T. Newly developed primary culture of rat visceral adipocytes and their in vitro characteristics. Cell Biol Int. 2006; 30:381–388.
Article27. Sordillo LM, Raphael W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet Clin North Am Food Anim Pract. 2013; 29:267–278.
Article28. Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin A in lipid metabolism and adipocyte lipolysis. IUBMB Life. 2004; 56:379–385.
Article29. Vaandrager AB. Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol Cell Biochem. 2002; 230:73–83.
Article30. Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, Magee MS, Hyslop T, Schulz S, Waldman SA. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011; 121:3578–3588.
Article31. Wiegand RC, Kato J, Huang MD, Fok KF, Kachur JF, Currie MG. Human guanylin: cDNA isolation, structure, and activity. FEBS Lett. 1992; 311:150–154.
Article32. Zachut M. Defining the adipose tissue proteome of dairy cows to reveal biomarkers related to peripartum insulin resistance and metabolic status. J Proteome Res. 2015; 14:2863–2871.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alteration of Nitric Oxide Synthase and Guanylyl Cyclase Activity in Rats with Ischemia/Reperfusion Renal Injury
- Four Cases of Postoperative Sclerosing Mesenteritis
- Sex-dependent Depot Differences in Adipose Tissue Development and Function; Role of Sex Steroids
- Diminished vascular guanylyl cyclase activity in deoxycorticosterone acetate-salt hypertension
- Cases of Postoperative Mesenteric Panniculitis