Clin Orthop Surg.
2016 Dec;8(4):465-474. 10.4055/cios.2016.8.4.465.
Clinical Usefulness of Long-term Application of Fentanyl Matrix in Chronic Non-Cancer Pain: Improvement of Pain and Physical and Emotional Functions
- Affiliations
-
- 1Department of Orthopedic Surgery, Guri Hospital, Hanyang University College of Medicine, Guri, Korea. hyparkys@hanyang.ac.kr
- 2Department of Physical Medicine and Rehabilitation, Korea University College of Medicine, Seoul, Korea.
- 3Department of Orthopedic Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Orthopedic Surgery, Kosin University Gospel Hospital, Kosin University School of Medicine, Busan, Korea.
- 5Department of Orthopedic Surgery, Dong-A University College of Medicine, Busan, Korea.
- 6Department of Orthopedic Surgery, Daejeon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Daejeon, Korea.
- 7Department of Rehabilitation, Konkuk University College of Medicine, Chungju, Korea.
- 8Department of Neurosurgery, Seoul Medical Center, Seoul, Korea.
- 9Department of Psychiatry and Behavioral Science, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2412331
- DOI: http://doi.org/10.4055/cios.2016.8.4.465
Abstract
- BACKGROUND
Opioids are recently recommended for those who do not gain adequate pain relief from the use of acetaminophen or nonsteroidal anti-inflammatory drugs. Medical opioids are administered in various routes, and transdermal opioid products that can make up for the weaknesses of the oral or intravenous products have been developed. This study is to evaluate the clinical usefulness of fentanyl matrix in terms of the long-term improvement in pain and physical and mental functions.
METHODS
This was a multicenter, open, prospective, observational study that was conducted in 54 institutions in Korea. Patients with non-cancerous chronic pain completed questionnaires, and investigators also completed questionnaires. A total of 1,355 subjects participated in this study, and 639 subjects completed the study. Subjects received transdermal fentanyl matrix (12 µg/hr, 25 µg/hr, or 50 µg/hr depending on the patient's response and demand). Subjects visited at 29 ± 7 days, 85 ± 14 days, and 169 ± 14 days after administration, respectively, to receive drug titration and fill out the questionnaires. The results were analyzed using the intention-to-treat (ITT) analysis, full analysis set (FAS), and per-protocol (PP) analysis. The FAS analysis included only 451 participants; the PP analysis, 160 participants; and the ITT analysis, 1,355 participants.
RESULTS
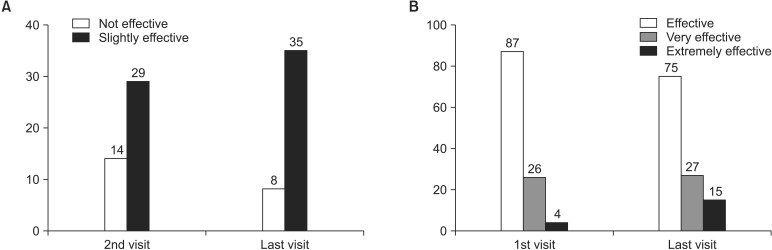
The intensity of pain measured by the Numeric Rating Scale decreased from 7.07 ± 1.78 to 4.93 ± 2.42. The physical assessment score and mental assessment score of the Short-Form Health Survey 12 improved from 28.94 ± 7.23 to 35.90 ± 10.25 and from 35.80 ± 11.76 to 42.52 ± 10.58, respectively. These differences were significant, and all the other indicators also showed improvement. Adverse events with an incidence of ≥ 1% were nausea, dizziness, vomiting, and pruritus.
CONCLUSIONS
The long-term administration of fentanyl matrix in patients with non-cancerous pain can reduce the intensity of pain and significantly improves activities of daily living and physical and mental capabilities.
MeSH Terms
Figure
Cited by 1 articles
-
Analgesic Efficacy and Safety of Prolonged-Release Oxycodone/Naloxone in Korean Patients with Chronic Pain from Spinal Disorders
Chang Ju Hwang, Sung Soo Chung, Kyu-Yeol Lee, Jae Hyup Lee, Seong-Hwan Moon, Jin-Hyok Kim, Kyu-Jung Cho, Jae-Sung Ahn, Dong-Soo Kim, Ye-Soo Park, Hye-Jeong Park
Clin Orthop Surg. 2018;10(1):33-40. doi: 10.4055/cios.2018.10.1.33.
Reference
-
1. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986; 3:S1–S226. PMID: 3461421.2. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians' (ASIPP) Guidelines. Pain Physician. 2008; 11(2 Suppl):S5–S62. PMID: 18443640.3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012; 13(8):715–724. PMID: 22607834.
Article4. Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998; 280(2):147–151. PMID: 9669787.5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006; 10(4):287–333. PMID: 16095934.
Article6. Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage. 2002; 23(2):131–137. PMID: 11844633.
Article7. Henderson JV, Harrison CM, Britt HC, Bayram CF, Miller GC. Prevalence, causes, severity, impact, and management of chronic pain in Australian general practice patients. Pain Med. 2013; 14(9):1346–1361. PMID: 23855874.
Article8. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007; 147(7):478–491. PMID: 17909209.
Article9. Grond S, Radbruch L, Lehmann KA. Clinical pharmacokinetics of transdermal opioids: focus on transdermal fentanyl. Clin Pharmacokinet. 2000; 38(1):59–89. PMID: 10668859.10. Muijsers RB, Wagstaff AJ. Transdermal fentanyl: an updated review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control. Drugs. 2001; 61(15):2289–2307. PMID: 11772140.11. Foley KM. The treatment of cancer pain. N Engl J Med. 1985; 313(2):84–95. PMID: 2582259.
Article12. Ashburn MA, Lipman AG. Management of pain in the cancer patient. Anesth Analg. 1993; 76(2):402–416. PMID: 8424523.13. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008; 16(5):405–416. PMID: 18837637.
Article14. Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006; 174(11):1589–1594. PMID: 16717269.
Article15. Yoo BW, Kim JH, Park YS. Effect of long-term treatment and efficacy of transdermal opioid patch in the chronic low back pain. Int J Pain. 2010; 1:15–20.16. Allan L, Hays H, Jensen NH, et al. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ. 2001; 322(7295):1154–1158. PMID: 11348910.17. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010; (1):CD006605. PMID: 20091598.
Article18. Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980; 302(2):123.
Article19. Kissin I. Long-term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety? J Pain Res. 2013; 6:513–529. PMID: 23874119.20. Lindley EM, Milligan K, Farmer R, Burger EL, Patel VV. Patient-controlled transdermal fentanyl versus intravenous morphine pump after spine surgery. Orthopedics. 2015; 38(9):e819–e824. PMID: 26375541.
Article21. Viscusi ER, Grond S, Ding L, Danesi H, Jones JB, Sinatra RS. A comparison of opioid-related adverse events with fentanyl iontophoretic transdermal system versus morphine intravenous patient-controlled analgesia in acute postoperative pain. Pain Manag. 2016; 6(1):19–24. PMID: 26376128.
Article22. Matsumoto S, Matsumoto K, Iida H. Transdermal fentanyl patch improves post-operative pain relief and promotes early functional recovery in patients undergoing primary total knee arthroplasty: a prospective, randomised, controlled trial. Arch Orthop Trauma Surg. 2015; 135(9):1291–1297. PMID: 26112273.
Article23. Hosseini H, Kargar S, Shiryazdi SM, Kargar S, Rezaie F, Neamatzadeh H. Fentanyl transdermal patch (Durogesic® DTRANS) for post abdominal laparotomy analgesia: a double blind randomized study. Minerva Chir. 2015; 70(6):401–408. PMID: 25517261.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Usefulness of Fentanyl Matrix Patch for the Patients with Chronic Low Back Pain
- Exercise-Induced Pain Reduction and Its Central Mechanism in Patients with Chronic Pain
- Two Cases of Fentanyl Intoxication Through Overusing Fentanyl Patch
- Long-term Placement of Epidural Catheter: A case report
- Psychiatric Treatment of Chronic Pain