Transl Clin Pharmacol.
2017 Mar;25(1):34-42. 10.12793/tcp.2017.25.1.34.
Development of an automated appendix generation system (ARGUS) for clinical study reports
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. yimds@catholic.ac.kr
- 2PIPET (Pharmacometrics Institute for Practical Education and Training), College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2411417
- DOI: http://doi.org/10.12793/tcp.2017.25.1.34
Abstract
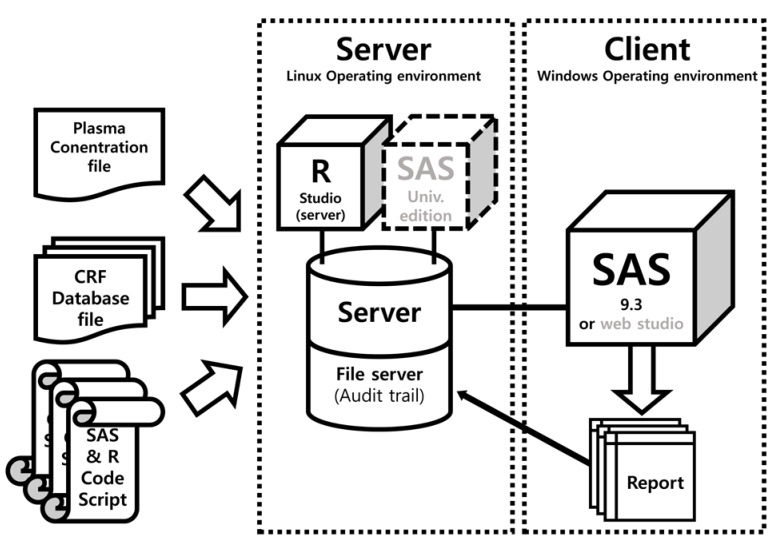
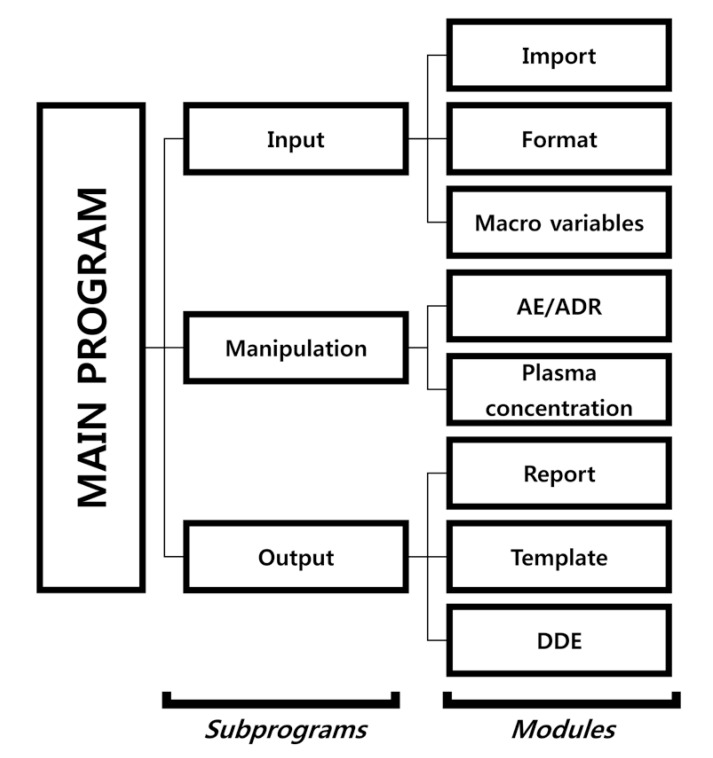
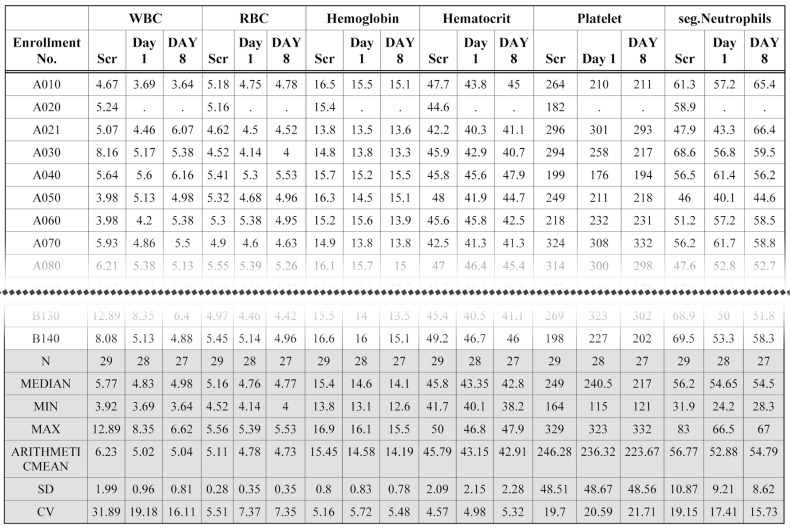
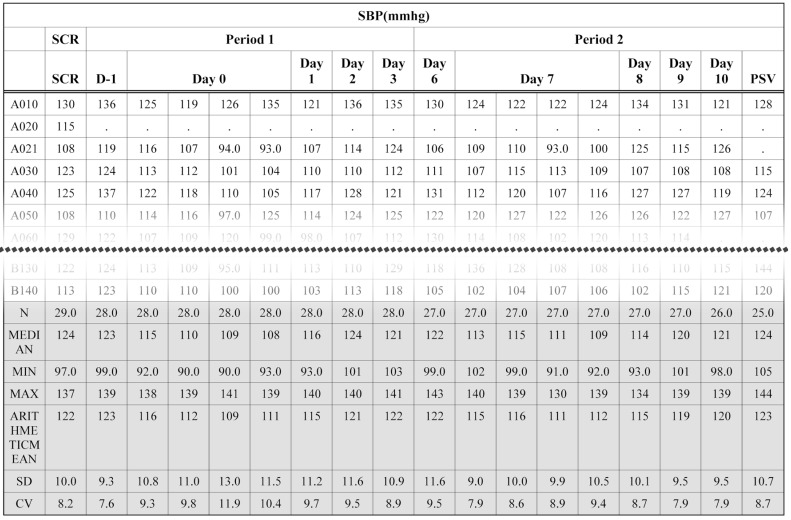
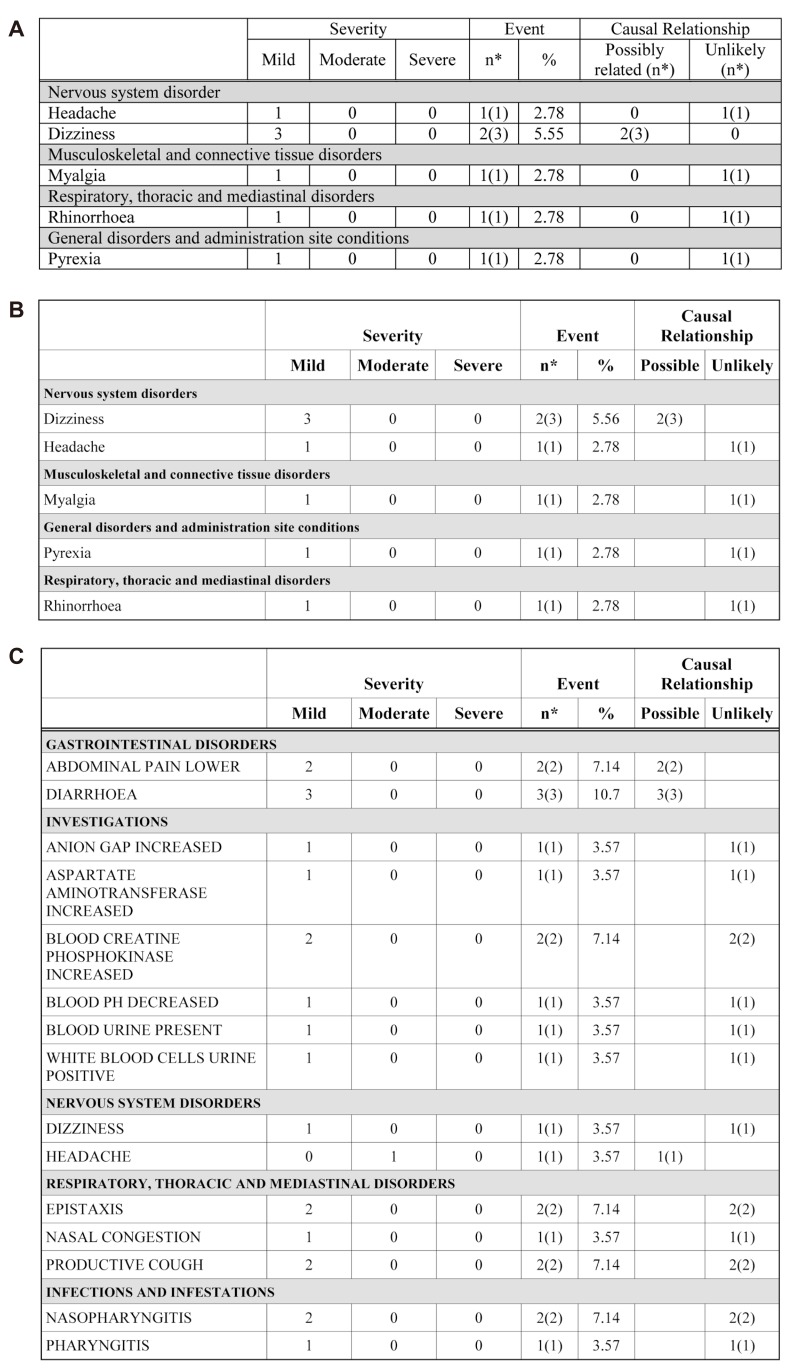
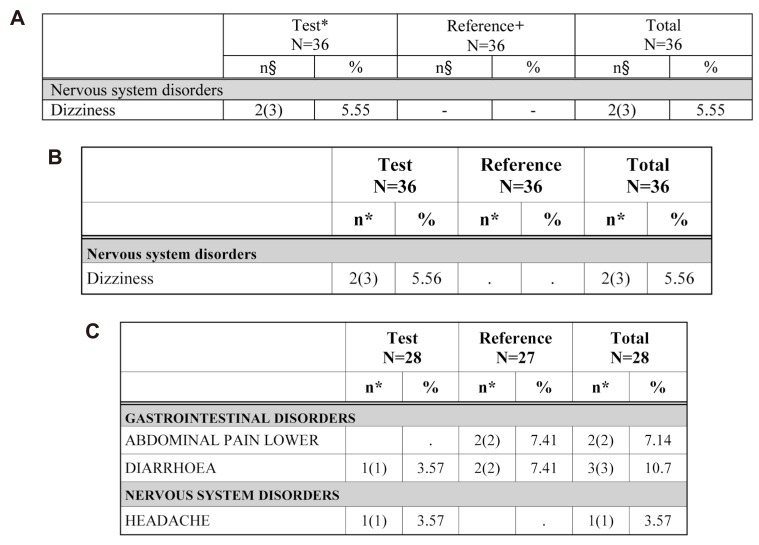
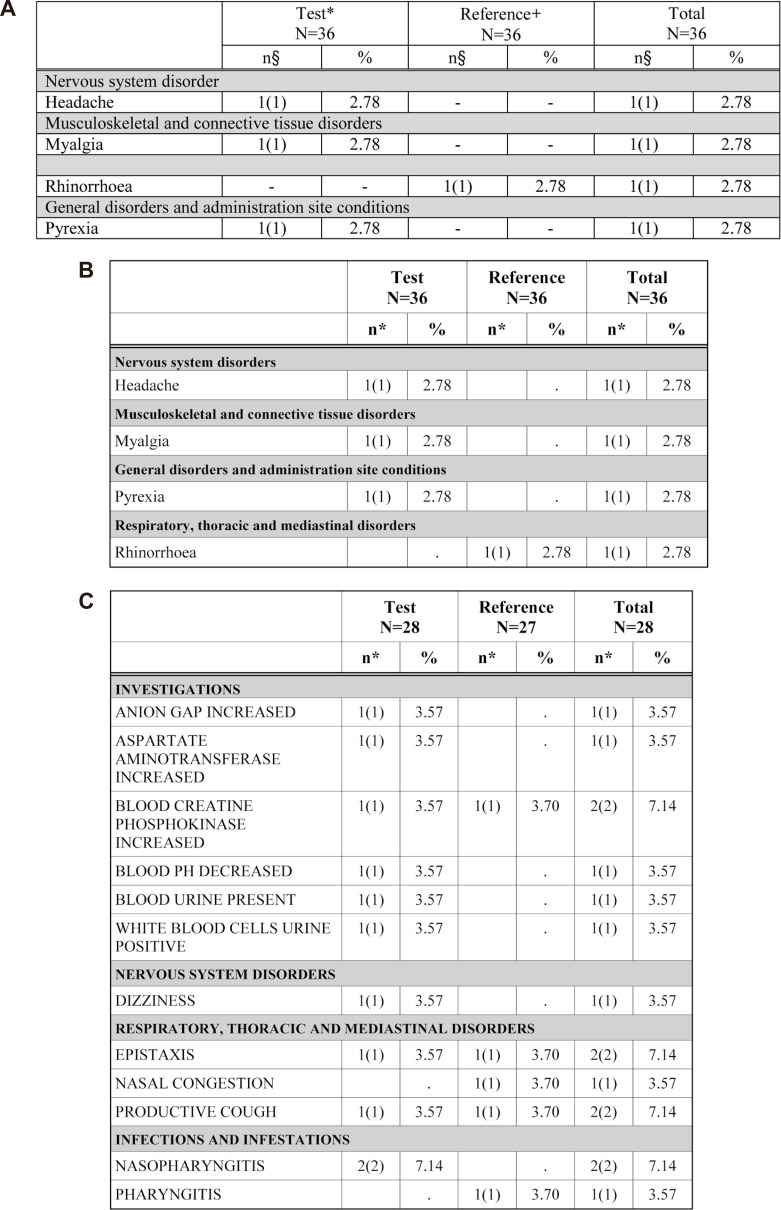
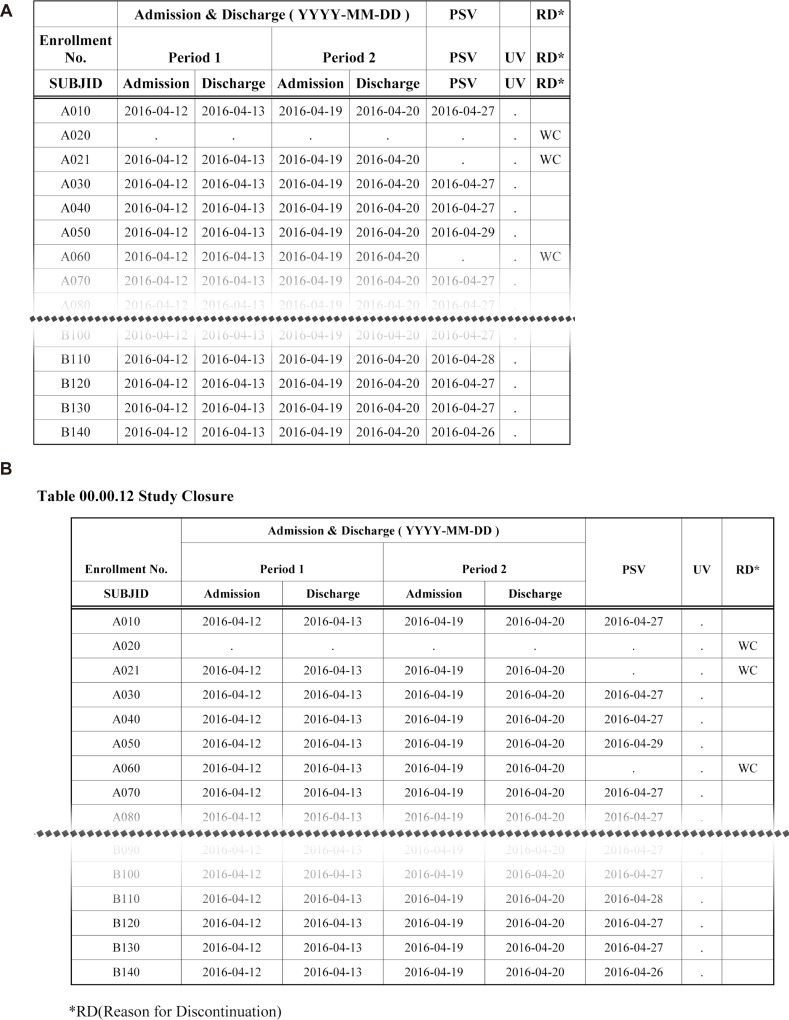
- Data handling and tabulation are a time-consuming job when writing appendices for clinical study reports. The authors have developed an automated appendix generation system (ARGUS) conforming to the CDISC/SDTM standard using SAS (version 9.3) and R (version 3.3.1: for PK plot generation). It consists of the one main program and three subprograms. The program runs to convert a database file into an appendix document with about 100 tables and plots in MS Word format within one min after pressing the submit button under common desktop environments. We found that tasks of constructing appendices for a typical 2×2 crossover design study that have taken our team about 8 days were completed within 6 or 7 hours using the ARGUS system.
Figure
Reference
-
1. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline - Structure and Content of Clinical Study Reports (E3). Accessed October 2 2016. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E3/E3_Guideline.pdf.2. Krishnankutty B, Bellary S, Kumar NB, Moodahadu LS. Data management in clinical research: An overview. Indian J Pharmacol. 2012; 44:168–172. DOI: 10.4103/0253-7613.93842. PMID: 22529469.
Article3. Wieseler B, Kerekes MF, Vervoelgyi V, McGauran N, Kaiser T. Impact of document type on reporting quality of clinical drug trials: a comparison of registry reports, clinical study reports, and journal publications. BMJ. 2012; 344:d8141. DOI: 10.1136/bmj.d8141. PMID: 22214759.
Article4. Hong MK, Yao HH, Pedersen JS, Peters JS, Costello AJ, Murphy DG, et al. Error rates in a clinical data repository: lessons from the transition to electronic data transfer--a descriptive study. BMJ Open. 2013; 3:pii:e002406. DOI: 10.1136/bmjopen-2012-002406.
Article5. Peng RD. Reproducible research and Biostatistics. Biostatistics. 2009; 10:405–408. DOI: 10.1093/biostatistics/kxp014. PMID: 19535325.
Article6. Gilmore J. Using Dynamic Data Exchange with Microsoft Word. Accessed August 1 2016. http://www2.sas.com/proceedings/sugi22/SYSARCH/PAPER308.PDF.7. Wood F, Schaefer P, Carolina N, Lewis R. Considerations in the Submission of Pharmacokinetics (PK) Data in an SDTM-Compliant Format. Accessed July 15 2017. http://www.pharmasug.org/proceedings/2012/DS/PharmaSUG-2012-DS10.pdf.8. Analysis Data Model (ADaM) Data Structure for Adverse Event Analysis. Accessed December 1 2016. https://www.cdisc.org/sites/default/files/.../adam/adam_ae_final_v1.pdf.9. Babcock G, York N. Dropping variables from a large SAS data set when all their values are missing. Accessed December 1 2016. http://www.lexjansen.com/nesug/nesug13/90_Final_Paper.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on Development of the Automated Critical Pathway for Patients with Cesarean Section
- Development Environment for Automatic Generation of Clinical Data Acquisition System
- Case Report of an Appendiceal Mucinous Neoplasm: A Rare Etiology for Appendicitis
- Colonoscopic Removal of an Inverted Appendix
- The Clinical Significance of Torsion of Appendix Testis in Acute Scrotum