Nat Prod Sci.
2018 Mar;24(1):71-78. 10.20307/nps.2018.24.1.71.
Optimization of Algerian Thymus fontanesii Boiss. & Reut Essential Oil Extraction by Electromagnetic Induction Heating
- Affiliations
-
- 1Laboratoire de Valorisation des Substances Naturelles, Université Djilali Bounaama de Khemis-Miliana, Route de Theniet El-Had, W. Aïn-Defla 44225, Algeria. lamia_sidali@hotmail.com
- 2Département Agro Bio Chem, Chimie Générale et Organique, Gembloux Agro Bio Tech Université de Liège, B-5030 Gembloux, Belgium.
- KMID: 2409613
- DOI: http://doi.org/10.20307/nps.2018.24.1.71
Abstract
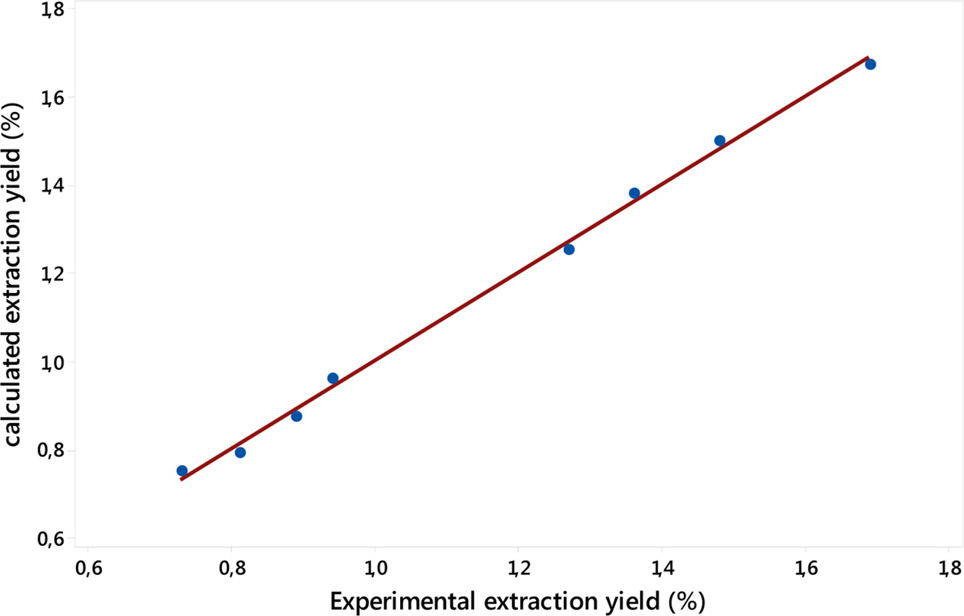
- The present study deals with the determination of optimal values of operating parameters such as the temperature of heating, the mass of the plant material and the volume of water leading to the best yield of electromagnetic induction (EMI) heating extraction of Algerian Thymus fontanesii essential oil. After an appropriate choice of the three critical variables, eight experiments leaded to a mathematical model as a first-degree polynomial presenting the response function (yield) in the relation to the operating parameters. From the retained model, we were able to calculate the average response, the different effects and their interactions. The maximum of essential oil recovery percentage relative to the initial mass of plant material was 1.69%, and was obtained at (140 ℃, 250 g and 4.5 L). The chemical composition of the Algerian T. fontanesii essential oil under the obtained optimal conditions (140 ℃, 250 g and 4.5 L), determined by GC/MS and GC/FID, reveled of the presence of major components such as: carvacrol (70.6 ± 0.1%), followed by p-cymene (8.2 ± 0.2%).
Keyword
Figure
Reference
-
References
(1). Morales R.., Stahl-Biskup E.., Sáez, F. The history botany and taxonomy of the genus Thymus. Thyme: the genus Thymus; Medicinal and Aromatic Plants; Taylor and Francis: U.K,. 2002. 1–43.(2). Nabavi S. M.., Marchese A.., Izadi M.., Curti V.., Daglia M.., Nabavi S. F.Food Chem. 2015. 173:339–347.(3). Barros L.., Heleno S. A.., Carvalho A. M.., Ferreira I. C.LWT-Food Sci. Technol. 2010. 43:544–550.(4). Zarzuelo A.., Crespo E.The medicinal and non-medicinal uses of thyme. In Thyme: the genus Thymus, CRC Press:. 2002.(5). Mann J.Phytochemistry. 1998. 48:585.(6). Casiglia S.., Bruno M.., Scandolera E.., Senatore F.Arab. J. Chem, In Press, 2015. In Press,. 2015.(7). Stahl-Biskup E.., Sáez F.Thyme: the genus Thymus; Medicinal and Aromatic Plants; Taylor and Francis: London,. 2002.(8). Ghannadi A.., Sajjadi S. E.., Kabouche A. Z.Naturforsch. C. 2004. 59:187–189.(9). Starmans D. A.., Nijhuis H. H.Trends Food Sci. Technol. 1996. 7:191–197.
Article(10). Wang L.., Weller C. L.Trends Food Sci. Technol. 2006. 17:300–312.(11). El Asbahani A.., Miladi K.., Badri W.., Sala M.., Aït Addi E. H.., Casabianca H.., El Mousadik A.., Hartmann D.., Jilale A.., Renaud F. N. R.., Elaissari A.Int. J. Pharm. 2015. 483:220–243.(12). Ammar A. H.., Zagrouba F.., Romdhane M.Flavour Frag. J. 2010. 25:503–507.(13). Adams R. P.Quadrupole mass spectra of compounds listed in order of their retention time on DB-5. Identification of essential oils components by gas chromatography: quadrupole mass spectroscopy; Allured Publishing Corporation: USA,. 2001. , 3rd ed.(14). Joulain D.., König, W. A. J. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. E.B. –Verlag Hambourg J.Nat. Prod.,. 1999. 62(8):1212–1213.(15). Babushok V. I.., Linstrom P. J.., Zenkevich I. G. J.Phys. Chem. Ref. Data. 2011. 40:043101.(16). Huiping L.., Guoqun Z.., Shanting N.., Yiguo L.Comput. Mater. Sci. 2007. 38:561–570.(17). Ravikumar K.., Krishnan S.., Ramalingam S.., Balu K.Dyes Pigm. 2007. 72:66–74.(18). Bekhechi C.., Bekkara F. A.., Abdelouahid D. E.., Tomi F.., Casanova J. J.Essent. Oil Res. 2007. 19:594–596.(19). Sidali L.., Brada M.., Fauconnier M. L.., Lognay G.., Heuskin S.PhytoChem Biosub J. 2017. 11:11.(20). Mouhi L.., Moghrani H.., Nasrallah N.., Amrane A.., Maachi R. J.Food Biochem. 2017. 41:1–9.(21). Dob T.., Dahmane D.., Benabdelkader T.., Chelghoum C.Pharm. Biol. 2006. 44:607–612.(22). Bailer J.., Aichinger T.., Hackl G.., de Hueber K.., Dachler M.Ind. Crops Prod. 2001. 14:229–239.(23). Desai M. A.., Parikh J.., De A. K.Modelling and optimization studies on extraction of lemongrass oil from Cymbopogon flexuosus (Steud.) Wats Chem. Eng. Res. Des. 2014. 92:793–803.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemical Composition and Antioxidant Activity of Algerian Juniperus Phoenicea Essential Oil
- RF Heating of Implants in MRI: Electromagnetic Analysis and Solutions
- Antifungal Activity of Clove Essential Oil and its Volatile Vapour Against Dermatophytic Fungi
- Presence of Two Apocarotenoids in Volatile Constituents of Onosma dichroanthum
- A Study Of Effect Of Pulsed Electromagnetic Fields On Osteogenesis In Rabbit Cranial Bone Defect