Endocrinol Metab.
2015 Sep;30(3):389-394. 10.3803/EnM.2015.30.3.389.
Acromegaly due to a Macroinvasive Plurihormonal Pituitary Adenoma and a Rectal Carcinoid Tumor
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Kyung Hee University School of Medicine, Seoul, Korea. igf1@unitel.co.kr
- 2Institute of Pathology, Helmholtz Zentrum Munchen, Neuherberg, Germany.
- KMID: 2407091
- DOI: http://doi.org/10.3803/EnM.2015.30.3.389
Abstract
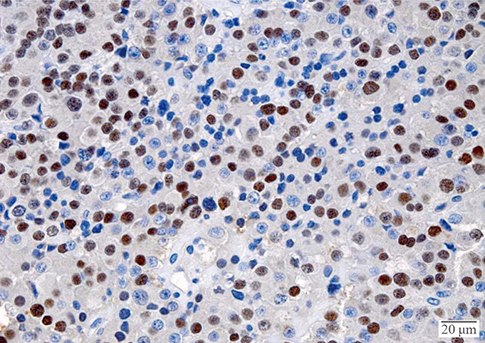
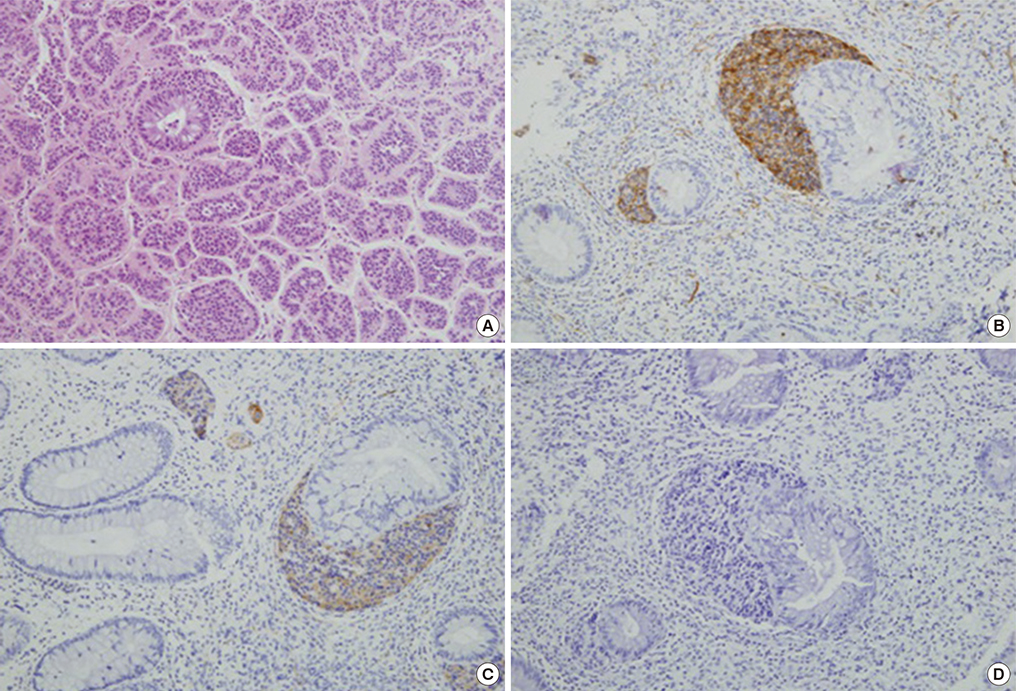
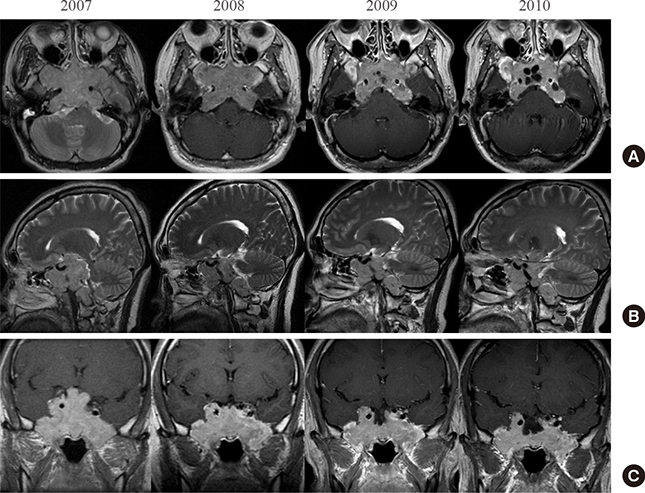
- A macroinvasive pituitary adenoma with plurihormonality usually causes acromegaly and hyperprolactinemia, and also accompanies with neurologic symptoms such as visual disturbances. However, its concurrent presentation with a rectal carcinoid tumor is rarely observed. This study reports the history, biochemical, colonoscopic and immunohistochemical results of a 48-year-old female with acromegaly and hyperprolactinemia. Despite the large size and invasive nature of the pituitary adenoma to adjacent anatomical structures, she did not complain of any neurologic symptoms such as visual disturbance or headache. Immunohistochemical staining of the surgical specimen from the pituitary adenoma revealed that the tumor cells were positive for growth hormone (GH), prolactin (PRL), and thyroid stimulating hormone (TSH). Staining for pituitary-specific transcription factor-1 (Pit-1) was shown to be strongly positive, which could have been possibly contributing to the plurihormonality of this adenoma. Colonoscopy found a rectal polyp that was identified to be a carcinoid tumor using immunohistochemical staining. A macroinvasive pituitary adenoma with concomitant rectal carcinoid tumor was secreting GH, PRL, and TSH, which were believed to be in association with over-expression of Pit-1. This is the first case report of double primary tumors comprising a plurihormonal pituitary macroadenoma and rectal carcinoid tumor.
Keyword
MeSH Terms
Figure
Reference
-
1. Ho DM, Hsu CY, Ting LT, Chiang H. Plurihormonal pituitary adenomas: immunostaining of all pituitary hormones is mandatory for correct classification. Histopathology. 2001; 39:310–319.2. Salehi F, Cohen S, Syro LV, Uribe H, Horvath E, Kovacs K, et al. Plurihormonality in pituitary adenomas associated with acromegaly. Endocr Pathol. 2006; 17:291–296.3. Pellegrini I, Barlier A, Gunz G, Figarella-Branger D, Enjalbert A, Grisoli F, et al. Pit-1 gene expression in the human pituitary and pituitary adenomas. J Clin Endocrinol Metab. 1994; 79:189–196.4. Osamura RY, Tahara S, Kurotani R, Sanno N, Matsuno A, Teramoto A. Contributions of immunohistochemistry and in situ hybridization to the functional analysis of pituitary adenomas. J Histochem Cytochem. 2000; 48:445–458.5. Yamada S, Takahashi M, Hara M, Hattori A, Sano T, Ozawa Y, et al. Pit-1 gene expression in human pituitary adenomas using the reverse transcription polymerase chain reaction method. Clin Endocrinol (Oxf). 1996; 45:263–272.6. Quintanilla-Martinez L, Kremer M, Specht K, Calzada-Wack J, Nathrath M, Schaich R, et al. Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am J Pathol. 2003; 162:1449–1461.7. Matsuno A, Teramoto A, Takekoshi S, Sanno N, Osamura RY, Kirino T. HGH, PRL, and ACTH gene expression in clinically nonfunctioning adenomas detected with nonisotopic in situ hybridization method. Endocr Pathol. 1995; 6:13–20.8. Kageyama K, Ikeda H, Nigawara T, Sakihara S, Suda T. Expression of adrenocorticotropic hormone, prolactin and transcriptional factors in clinically nonfunctioning pituitary adenoma. Endocr J. 2007; 54:961–968.9. Remenyi A, Tomilin A, Scholer HR, Wilmanns M. Differential activity by DNA-induced quarternary structures of POU transcription factors. Biochem Pharmacol. 2002; 64:979–984.10. Roche C, Rasolonjanahary R, Thirion S, Goddard I, Fusco A, Figarella-Branger D, et al. Inactivation of transcription factor pit-1 to target tumoral somatolactotroph cells. Hum Gene Ther. 2012; 23:104–114.11. Yin Z, Williams-Simons L, Rawahneh L, Asa S, Kirschner LS. Development of a pituitary-specific cre line targeted to the Pit-1 lineage. Genesis. 2008; 46:37–42.12. DeFranco DB. Editorial: molecular endocrinology articles in the spotlight for August 2012. Mol Endocrinol. 2012; 26:1251.13. Lopes MB. Growth hormone-secreting adenomas: pathology and cell biology. Neurosurg Focus. 2010; 29:E2.14. Corenblum B, Sirek AM, Horvath E, Kovacs K, Ezrin C. Human mixed somatotrophic and lactotrophic pituitary adenomas. J Clin Endocrinol Metab. 1976; 42:857–863.15. Felix IA, Horvath E, Kovacs K, Smyth HS, Killinger DW, Vale J. Mammosomatotroph adenoma of the pituitary associated with gigantism and hyperprolactinemia. A morphological study including immunoelectron microscopy. Acta Neuropathol. 1986; 71:76–82.16. Horvath E, Kovacs K, Singer W, Smyth HS, Killinger DW, Erzin C, et al. Acidophil stem cell adenoma of the human pituitary: clinicopathologic analysis of 15 cases. Cancer. 1981; 47:761–771.17. Kreutzer J, Vance ML, Lopes MB, Laws ER Jr. Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J Clin Endocrinol Metab. 2001; 86:4072–4077.18. Lloyd RV, Gikas PW, Chandler WF. Prolactin and growth hormone-producing pituitary adenomas. An immunohistochemical and ultrastructural study. Am J Surg Pathol. 1983; 7:251–260.19. Saeger W, Ludecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007; 156:203–216.20. Saeger W, Ludecke B, Ludecke DK. Clinical tumor growth and comparison with proliferation markers in non-functioning (inactive) pituitary adenomas. Exp Clin Endocrinol Diabetes. 2008; 116:80–85.21. Melmed S. Extrapituitary acromegaly. Endocrinol Metab Clin North Am. 1991; 20:507–518.22. Faglia G, Arosio M, Bazzoni N. Ectopic acromegaly. Endocrinol Metab Clin North Am. 1992; 21:575–595.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Immunohistochemical and Clinical Characteristics in Pituitary Adenoma with Acromegaly
- A Case of Acromegaly Caused by Mixed Gangliocytoma-Adenoma of the Pituitary Gland
- An Epidemiologic Study on Pituitary Diseas
- Mixed Gangliocytoma-Pituitary Adenoma: A case report
- Two Cases of Acromegaly with Empty Sella