Endocrinol Metab.
2015 Sep;30(3):352-360. 10.3803/EnM.2015.30.3.352.
Factors Associated with Glycemic Variability in Patients with Type 2 Diabetes: Focus on Oral Hypoglycemic Agents and Cardiovascular Risk Factors
- Affiliations
-
- 1Department of Internal Medicine, Jeju National University Hospital, Jeju, Korea. okdom@jejunu.ac.kr
- 2Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea.
- KMID: 2407087
- DOI: http://doi.org/10.3803/EnM.2015.30.3.352
Abstract
- BACKGROUND
The role of glycemic variability (GV) in development of cardiovascular diseases remains controversial, and factors that determine glucose fluctuation in patients with diabetes are unknown. We investigated relationships between GV indices, kinds of oral hypoglycemic agents (OHAs), and cardiovascular risk factors in patients with type 2 diabetes mellitus (T2DM).
METHODS
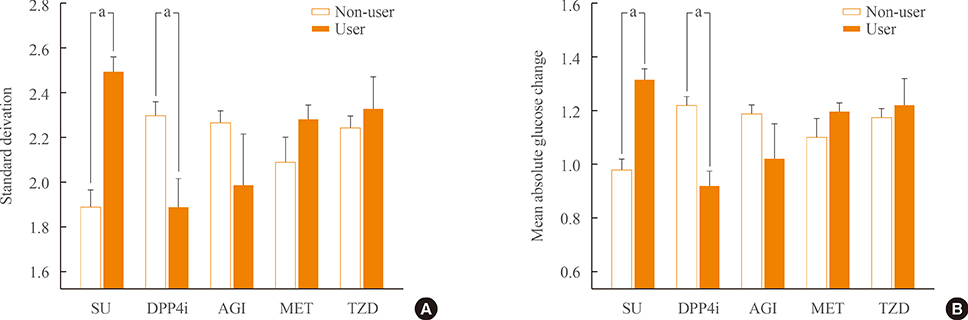
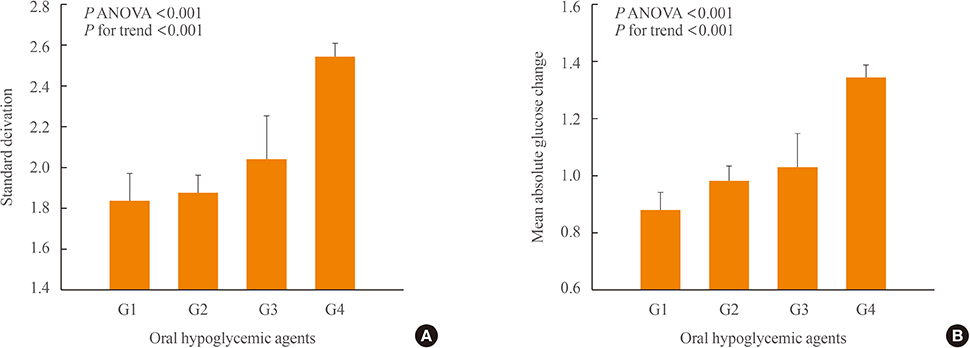
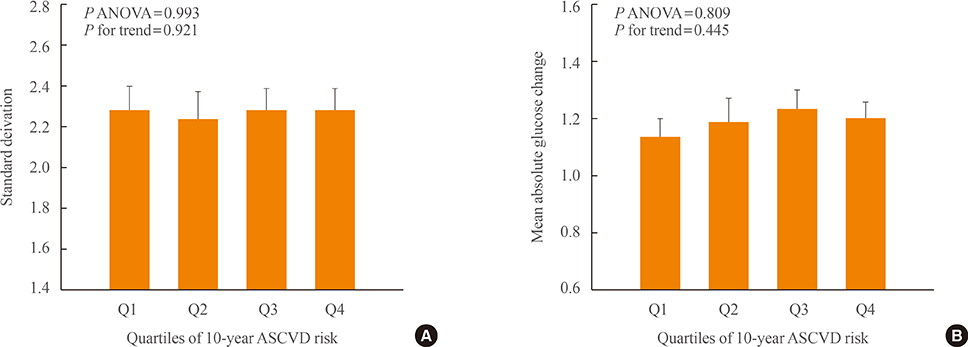
We analyzed 209 patients with T2DM. The GV index (standard deviation [SD] and mean absolute glucose change [MAG]) were calculated from 7-point self-monitoring of blood glucose profiles. The patients were classified into four groups according to whether they take OHAs known as GV-lowering (A) and GV-increasing (B): 1 (A only), 2 (neither), 3 (both A and B), and 4 (B only). The 10-year risk for atherosclerotic cardiovascular disease (ASCVD) was calculated using the Pooled Cohort Equations.
RESULTS
GV indices were significantly higher in patients taking sulfonylureas (SUs), but lower in those taking dipeptidyl peptidase-4 inhibitors. In hierarchical regression analysis, the use of SUs remained independent correlates of the SD (beta=0.209, P=0.009) and MAG (beta=0.214, P=0.011). In four OHA groups, GV indices increased progressively from group 1 to group 4. However, these did not differ according to quartiles of 10-year ASCVD risk.
CONCLUSION
GV indices correlated significantly with the use of OHAs, particularly SU, and differed significantly according to combination of OHAs. However, cardiovascular risk factors and 10-year ASCVD risk were not related to GV indices. These findings suggest that GV is largely determined by properties of OHAs and not to cardiovascular complications in patients with T2DM.
Keyword
MeSH Terms
Figure
Reference
-
1. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414:813–820.2. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28:103–117.3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853.4. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.5. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003; 52:2795–2804.6. Horvath EM, Benko R, Kiss L, Muranyi M, Pek T, Fekete K, et al. Rapid 'glycaemic swings' induce nitrosative stress, activate poly(ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia. 2009; 52:952–961.7. Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010; 38:838–842.8. Wang JS, Lin SD, Lee WJ, Su SL, Lee IT, Tu ST, et al. Effects of acarbose versus glibenclamide on glycemic excursion and oxidative stress in type 2 diabetic patients inadequately controlled by metformin: a 24-week, randomized, open-label, parallel-group comparison. Clin Ther. 2011; 33:1932–1942.9. Guerci B, Monnier L, Serusclat P, Petit C, Valensi P, Huet D, et al. Continuous glucose profiles with vildagliptin versus sitagliptin in add-on to metformin: results from the randomized Optima study. Diabetes Metab. 2012; 38:359–366.10. Kohnert KD, Augstein P, Zander E, Heinke P, Peterson K, Freyse EJ, et al. Glycemic variability correlates strongly with postprandial beta-cell dysfunction in a segment of type 2 diabetic patients using oral hypoglycemic agents. Diabetes Care. 2009; 32:1058–1062.11. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005; 54:1–7.12. DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001; 161:397–405.13. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003; 290:486–494.14. NAVIGATOR Study Group. Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010; 362:1463–1476.15. Andrus B, Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014; 63(25 Pt A):2886.16. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005; 143:559–569.17. Yamazaki M, Hasegawa G, Majima S, Mitsuhashi K, Fukuda T, Iwase H, et al. Effect of repaglinide versus glimepiride on daily blood glucose variability and changes in blood inflammatory and oxidative stress markers. Diabetol Metab Syndr. 2014; 6:54.18. Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, et al. HbA(1)(c) and mean blood glucose show stronger associations with cardiovascular disease risk factors than do postprandial glycaemia or glucose variability in persons with diabetes: the A1C-Derived Average Glucose (ADAG) study. Diabetologia. 2011; 54:69–72.19. Jin SM, Kim TH, Bae JC, Hur KY, Lee MS, Lee MK, et al. Clinical factors associated with absolute and relative measures of glycemic variability determined by continuous glucose monitoring: an analysis of 480 subjects. Diabetes Res Clin Pract. 2014; 104:266–272.20. Bradescu OM, Ionescu-Tirgoviste C. Correlates of the diurnal plasma glucose variability in non-insulin-treated type 2 diabetic patients. Proc Rom Acad Ser B. 2008; 1:53–58.21. Greenbaum CJ, Mandrup-Poulsen T, McGee PF, Battelino T, Haastert B, Ludvigsson J, et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008; 31:1966–1971.22. Raz I, Wilson PW, Strojek K, Kowalska I, Bozikov V, Gitt AK, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009; 32:381–386.23. Siegelaar SE, Kerr L, Jacober SJ, Devries JH. A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care. 2011; 34:855–857.24. Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970; 19:644–655.25. Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther. 2009; 11:551–565.26. Kohnert KD, Heinke P, Fritzsche G, Vogt L, Augstein P, Salzsieder E. Evaluation of the mean absolute glucose change as a measure of glycemic variability using continuous glucose monitoring data. Diabetes Technol Ther. 2013; 15:448–454.27. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.