J Pathol Transl Med.
2017 Nov;51(6):588-593. 10.4132/jptm.2017.08.10.
Aggressive Supratentorial Ependymoma, RELA Fusion-Positive with Extracranial Metastasis: A Case Report
- Affiliations
-
- 1Department of Pathology, Seoul National University Children’s Hospital, Seoul, Korea. shparknp@snu.ac.kr
- 2Department of Neurosurgery, Seoul National University Children’s Hospital, Seoul, Korea.
- 3Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, Korea.
- KMID: 2396542
- DOI: http://doi.org/10.4132/jptm.2017.08.10
Abstract
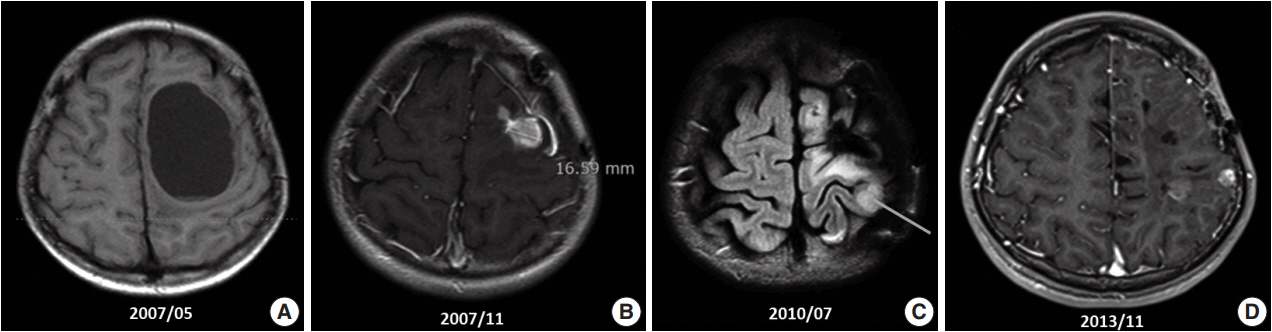
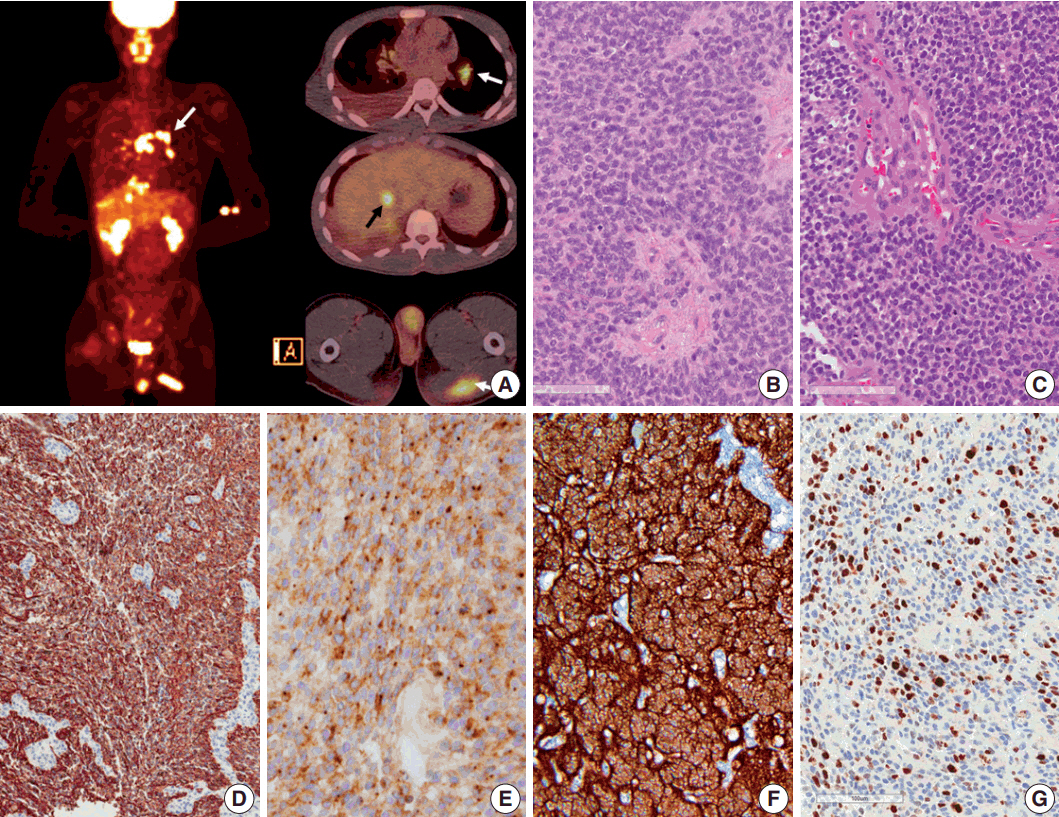
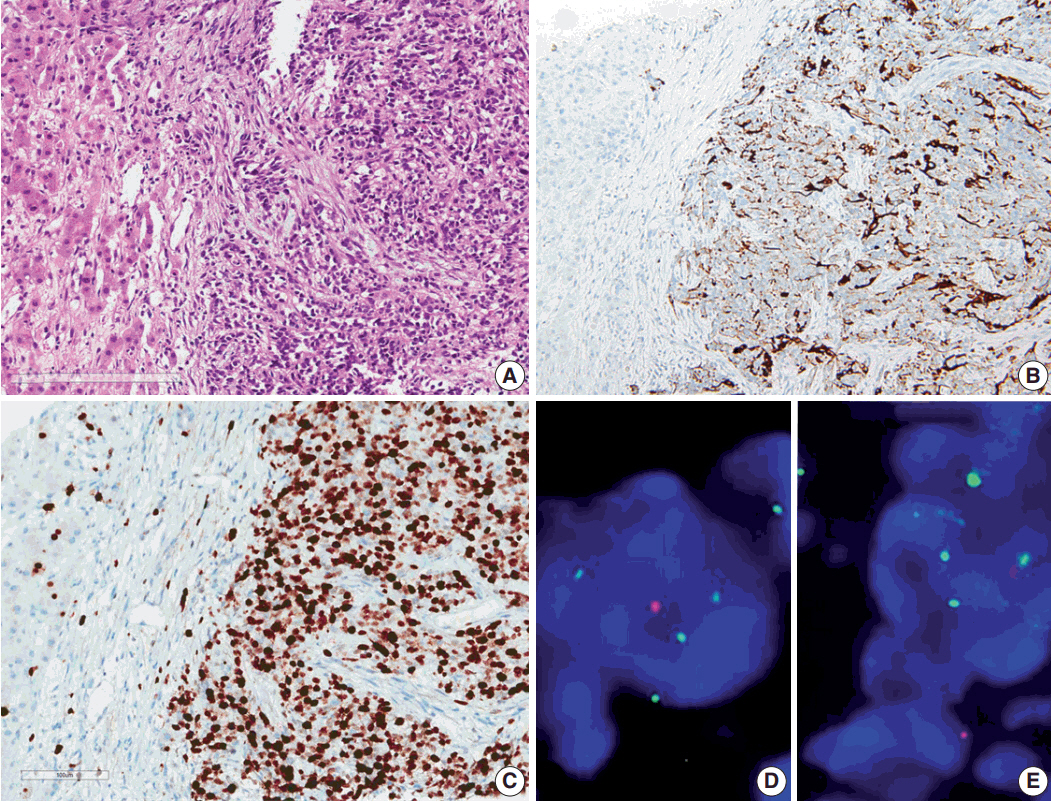
- Ependymoma is the third most common pediatric primary brain tumor. Ependymomas are categorized according to their locations and genetic abnormalities, and these two parameters are important prognostic factors for patient outcome. For supratentorial (ST) ependymomas, RELA fusion-positive ependymomas show a more aggressive behavior than YAP1 fusion-positive ependymomas. Extracranial metastases of intra-axial neuroepithelial tumors are extremely rare. In this paper, we report a case of aggressive anaplastic ependymoma arising in the right frontoparietal lobe, which had genetically 1q25 gain, CDKN2A homozygous deletion, and L1CAM overexpression. The patient was a 10-year-old boy who underwent four times of tumor removal and seven times of gamma knife surgery. Metastatic loci were scalp and temporalis muscle overlying primary operation site, lung, liver, buttock, bone, and mediastinal lymph nodes. He had the malignancy for 10 years and died. This tumor is a representative case of RELA fusion-positive ST ependymoma, showing aggressive behavior.
Keyword
MeSH Terms
Figure
Reference
-
1. Chhabda S, Carney O, D’Arco F, Jacques TS, Mankad K. The 2016 World Health Organization Classification of tumours of the central nervous system: what the paediatric neuroradiologist needs to know. Quant Imaging Med Surg. 2016; 6:486–9.
Article2. Perry A, Wesseling P. Histologic classification of gliomas. Handb Clin Neurol. 2016; 134:71–95.
Article3. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016; 18(Suppl_5):v1–v75.
Article4. Pachella LA, Kamiya-Matsuoka C, Lee EL, Olar A, Yung WK. Supratentorial extraventricular anaplastic ependymoma with extracranial metastasis. J Clin Neurosci. 2015; 22:605–7.
Article5. Davis MJ, Hasan F, Weinreb I, Wallace MC, Kiehl TR. Extraventricular anaplastic ependymoma with metastasis to scalp and neck. J Neurooncol. 2011; 104:599–604.
Article6. Chao MM, Packer RJ, Myseros JS, Rood BR. Isolated extracranial recurrence of anaplastic ependymoma. Pediatr Blood Cancer. 2011; 56:317–8.
Article7. Kumar P, Rastogi N, Jain M, Chhabra P. Extraneural metastases in anaplastic ependymoma. J Cancer Res Ther. 2007; 3:102–4.8. Itoh J, Usui K, Itoh M, Hashizume Y. Extracranial metastases of malignant ependymoma: case report. Neurol Med Chir (Tokyo). 1990; 30:339–45.9. Ferracini R, Manetto V, Poletti V, Giangasparo F. A cerebral ependymoma with extracranial metastases. Tumori. 1984; 70:389–92.
Article10. Wentworth P, Birdsell DC. Intracranial ependymoma with extracranial metastases. J Neurosurg. 1966; 25:648–51.
Article11. Maass L. Occipital ependymoma with extracranial metastases. J Neurosurg. 1954; 11:413–21.
Article12. Korshunov A, Golanov A, Sycheva R, Timirgaz V. The histologic grade is a main prognostic factor for patients with intracranial ependymomas treated in the microneurosurgical era: an analysis of 258 patients. Cancer. 2004; 100:1230–7.13. Korshunov A, Witt H, Hielscher T, et al. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol. 2010; 28:3182–90.
Article14. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015; 27:728–43.
Article15. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–20.
Article16. Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric brain tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol. 2015; 33:2986–98.
Article17. Nobusawa S, Hirato J, Yokoo H. Molecular genetics of ependymomas and pediatric diffuse gliomas: a short review. Brain Tumor Pathol. 2014; 31:229–33.
Article18. Ernestus RI, Schroder R, Stutzer H, Klug N. Prognostic relevance of localization and grading in intracranial ependymomas of childhood. Childs Nerv Syst. 1996; 12:522–6.
Article19. McGuire CS, Sainani KL, Fisher PG. Both location and age predict survival in ependymoma: a SEER study. Pediatr Blood Cancer. 2009; 52:65–9.
Article20. Rickert CH. Extraneural metastases of paediatric brain tumours. Acta Neuropathol. 2003; 105:309–27.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pure Cortical Ependymoma With RELA-Fusion Positive in a Young Adult
- Extracranial Metastasis of Supratentorial Ependymoma without Recurrence of Primary Focus
- Supratentorial Anaplastic Ependymoma Mimicking an Extra-Axial Tumor: A Case Report
- Supratentorial Cortical Ependymoma in a 21-Month-Old Boy
- A Case of Supratentorial Intra-axial Ependymoma Showing Exophytic Growth