J Korean Assoc Oral Maxillofac Surg.
2017 Oct;43(5):299-304. 10.5125/jkaoms.2017.43.5.299.
Rabbit submandibular salivary gland replantation
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Seoul National University, Seoul, Korea. leejongh@snu.ac.kr
- 2Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Sana'a University, Sana'a, Yemen.
- 3The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, China.
- 4Dental Research Institute, Seoul National University, Seoul, Korea.
- 5Oral Cancer Center, Seoul National University Dental Hospital, Seoul, Korea.
- KMID: 2393594
- DOI: http://doi.org/10.5125/jkaoms.2017.43.5.299
Abstract
OBJECTIVES
To test the feasibility of submandibular salivary gland (SMG) replantation techniques and the survival of the replanted glands. Such a study can provide a rationale for later allotransplantation procedures, along with implementation of conventional and advanced immunosuppression therapy.
MATERIALS AND METHODS
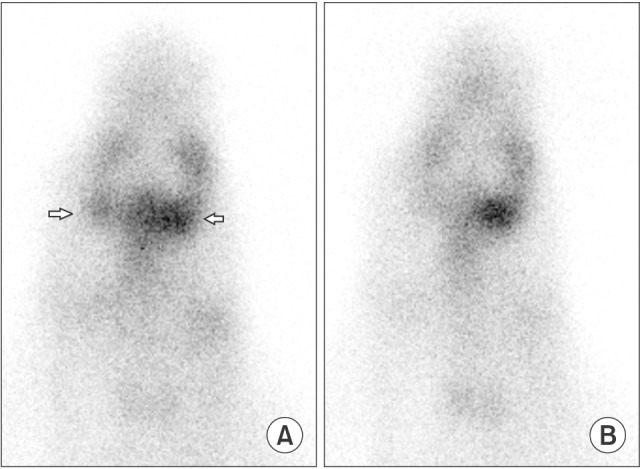
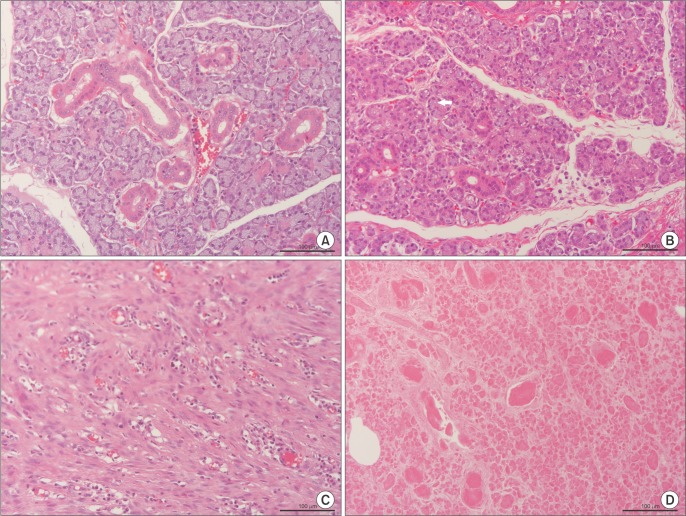
Six SMG replantations were performed in New Zealand white rabbits. One week postoperatively, (99m)Tc scintigraphy was performed and the uptake ratio and salivary excretion fraction were calculated. Two to four weeks later, submandibular glands were excised, fixed, and stained with H&E for histomorphometric evaluation.
RESULTS
Intraoperatively, all glands showed patent blood perfusion except gland 5. Positive tracer uptake and saliva excretion were documented by scintigraphy. On excision, all of the glands except glands 4 and 5 looked viable, with a red color and patent pedicles. Gland 4 was infected and filled with creamy pus, while gland 5 looked pale and necrotic. Histologically, glands 1, 2, 3, and 6 had preserved normal glandular tissue with slight variations from the contralateral normal glands, as their parenchyma was composed of mildly atrophic acini.
CONCLUSION
Four out of six replanted SMGs successfully survived. The glands maintained good viability and function. Such success depends on safe harvesting, short anastomosis time, and strict control of infection.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
FK506 immunosuppression for submandibular salivary gland allotransplantation in rabbit
Akram Abdo Almansoori, Namuun Khentii, Kyung Won Ju, Bongju Kim, Soung Min Kim, Jong-Ho Lee
J Korean Assoc Oral Maxillofac Surg. 2020;46(3):197-203. doi: 10.5125/jkaoms.2020.46.3.197.
Reference
-
1. Hanley O, Leech M. Reduction of xerostomia in head and neck cancer patients. A critical review of the literature. Radiography. 2016; 22(Suppl 1):S57–S63.
Article2. Mercadante V, Al Hamad A, Lodi G, Porter S, Fedele S. Interventions for the management of radiotherapy-induced xerostomia and hyposalivation: a systematic review and meta-analysis. Oral Oncol. 2017; 66:64–74. PMID: 28249650.
Article3. Kumar PA, Macleod AM, O'Brien BM, Hickey MJ, Knight KR. Microvascular submandibular gland transfer for the management of xerophthalmia; an experimental study. Br J Plast Surg. 1990; 43:431–436. PMID: 1697488.4. Spiegel JH, Deschler DG, Cheney ML. Microvascular transplantation and replantation of the rabbit submandibular gland. Arch Otolaryngol Head Neck Surg. 2001; 127:991–996. PMID: 11493212.
Article5. Hakim SG, Benedek GA, Su YX, Jacobsen HC, Klinger M, Dendorfer A, et al. Radioprotective effect of lidocaine on function and ultrastructure of salivary glands receiving fractionated radiation. Int J Radiat Oncol Biol Phys. 2012; 82:e623–e630. PMID: 22245203.
Article6. Liu XJ, Li M, Su JZ, Wang Z, Xie Z, Yu GY. Carbachol improves the secretion of transplanted submandibular glands during the latent period after microvascular autologous transplantation for severe keratoconjunctivitis sicca. Int J Oral Maxillofac Surg. 2016; 45:1273–1279. PMID: 27094607.
Article7. Su WF, Jen YM, Chen SG, Nieh S, Wang CH. Microvascular transplantation and replantation of the dog submandibular gland. Eur Arch Otorhinolaryngol. 2006; 263:490–494. PMID: 16362265.
Article8. Lee JS, Hamilton MG, Zabramski JM. Variations in the anatomy of the rabbit cervical carotid artery. Stroke. 1994; 25:501–503. PMID: 8303763.
Article9. Sieg P, Geerling G, Kosmehl H, Lauer I, Warnecke K, von Domarus H. Microvascular submandibular gland transfer for severe cases of keratoconjunctivitis sicca. Plast Reconstr Surg. 2000; 106:554–560. PMID: 10987460.
Article10. Bohuslavizki KH, Brenner W, Klutmann S, Hübner RH, Lassmann S, Feyerabend B, et al. Radioprotection of salivary glands by amifostine in high-dose radioiodine therapy. J Nucl Med. 1998; 39:1237–1242. PMID: 9669401.11. Bohuslavizki KH, Klutmann S, Jenicke L, Brenner W, Feyerabend B, Henze E, et al. Radioprotection of salivary glands by S-2-(3-aminopropylamino)-ethylphosphorothioic (amifostine) obtained in a rabbit animal model. Int J Radiat Oncol Biol Phys. 1999; 45:181–186. PMID: 10477022.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recurrent Sialolithiasis on Remnant Wharton's Duct Following Submandibular Gland Resection
- Salivary Duct Carcinoma: 2 Case Reports
- A Case of Multiple Sialoliths in Sublingual Gland Misdiagnosed as Sialoliths in Submandibular Gland
- A Case of Unilateral Absence of the Submandibular Gland Secondary to Sialolithiasis
- A giant sialolith in a wharton's duct: A case report